A trial of chemotherapy before chemoradiotherapy for cervical cancer (INTERLACE)
Cancer type:
Status:
Phase:
This trial looked at having carboplatin and paclitaxel chemotherapy before  for cervical cancer. It was for people whose cancer couldn’t be removed with surgery.
for cervical cancer. It was for people whose cancer couldn’t be removed with surgery.
The trial was supported by Cancer Research UK. It was open for people to join between 2012 and 2022. The team presented the first results at a conference in 2023.
More about this trial
This trial was for women with cervical cancer that hadn’t spread but couldn’t be removed with surgery.
When this trial was done, doctors usually treated women in this situation with radiotherapy and chemotherapy at the same time. This is called chemoradiotherapy or chemoradiation.
Researchers hoped that having chemotherapy before chemoradiotherapy would work better.
The people taking part in this trial were put into a treatment group at random.
Half had radiotherapy and cisplatin at the same time (chemoradiotherapy). This was the standard treatment. They had this treatment for 5 weeks.
Half had carboplatin and paclitaxel chemotherapy for 6 weeks to begin with. Then 5 weeks of chemoradiotherapy with cisplatin as usual.
The main aim of this trial was to see if it is better to have chemotherapy before chemoradiotherapy or not.
Summary of results
This results so far show that it is better to have chemotherapy before chemoradiotherapy. This is for cervical cancer that has not spread but can’t be removed with surgery.
Results
A total of 500 people from 5 different countries took part in this trial. This included 380 people from the UK. They were put into 1 of 2 treatment groups:
- 250 had standard chemoradiotherapy
- 250 had chemotherapy followed by chemoradiotherapy
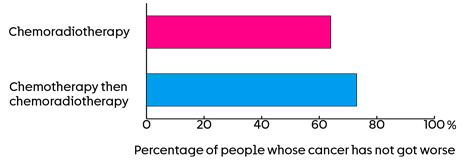
The team are looking at the number of people whose cancer has not grown, 5 years after joining the trial. The results so far show it is:
- 64 out of every 100 people (64%) who had standard chemoradiotherapy
- 73 out of every 100 people (73%) who had chemotherapy followed by chemoradiotherapy
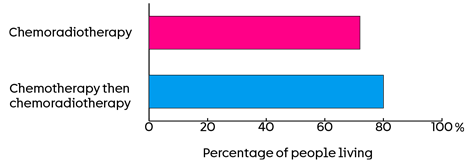
They are also looking at the number of people living, 5 years after joining the trial. The results so far show it is:
- 72 out of every 100 people (72%) who had standard chemoradiotherapy
- 80 out of every 100 people (80%) who had chemotherapy followed by chemoradiotherapy
Side effects
Most people taking part had at least one side effect from treatment. Many of these were mild or didn’t last long. But some people had more severe side effects:
- just under 5 out of 10 people (48%) who had chemoradiotherapy
- just under 6 out of 10 people (59%) who had chemotherapy and chemoradiotherapy.
The most common of these side effects in both groups were:
- diarrhoea
- extreme tiredness (fatigue)
- muscle weakness
- joint pain
In the chemotherapy and chemoradiotherapy group, the most common side effect was a drop in white blood cells causing an increased risk of infection (neutropenia).
We have more information about the side effects of:
Conclusion
The trial team concluded that it is better to have chemotherapy before chemoradiotherapy. They recommended that this becomes the new standard treatment for cervical cancer that hasn’t spread but can’t be removed with surgery.
More detailed information
There is more information about this research in the reference below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
LBA8 A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer: The GCIG INTERLACE trial
M. McCormack and others
Annals of Oncology, 2023. Volume 34, supplement 2, S1276.
Where this information comes from
We have based this summary on the information in the link above. As far as we are aware, the full results have not been reviewed by independent specialists ( ) or published in a medical journal yet. We have not analysed the data ourselves.
) or published in a medical journal yet. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Mary McCormack
Supported by
Cancer Research UK
Experimental Cancer Medicine Centre (ECMC)
Gynecologic Cancer InterGroup
NIHR Clinical Research Network: Cancer
National Cancer Research Institute (NCRI)
University College London (UCL)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040