Abstract
How do we develop a stable and coherent self-concept in contemporary times? Susan Harter’s original work, The Construction of Self (1999; 2012), argues that cognitive and social processes are building blocks for developing a coherent sense of self, resulting in self-concept clarity across various domains in life (e.g., [pro-]social, academic, and physical). Here, we show how this framework guides and can benefit from recent findings on (1) the prolonged and nonlinear structural brain development during childhood and adolescence, (2) insights from developmental neuroimaging studies using self-concept appraisal paradigms, (3) genetic and environmental influences on behavioral and neural correlates of self-concept development, and (4) youth’s perspectives on self-concept development in the context of 21st century global challenges. We examine how neuroscience can inform theory by testing several compelling questions related to stability versus change of neural, behavioral, and self-report measures and we reflect on the meaning of variability and change/growth.
Introduction
Adolescence refers to the time between childhood and adulthood, when individuals are approximately 10–24 years and is marked by a transition in how young people define their roles in society (Sawyer et al., 2018). During adolescence, young people broaden their social world outside the family context, adopt more complex identities, and develop perspectives on societal issues such as social inequality, education, and policy (Choudhury et al., 2023). Anthropologists who studied adolescence in different societies confirmed that all cultures have some form of adolescence, but there are differences in the timing and the length of adolescence (Schlegel & Barry, 1991). The definition of adolescence has also changed within our Western society across time, where scholars have argued that adolescence and the transition in emerging adulthood lengthens when the complexity of the society (e.g., in terms of technological and industrial development) increases (Arnett, 2000; Arnett et al., 2014). Most recently, adolescents who grow up in the 21st century deal with multiple offline and online information flows (Falk & Bassett, 2017), as well as multiple global crises, such as the recent COVID-19 pandemic, increasing social inequalities, global political tensions, and climate change (Choudhury et al., 2023).
This review addresses the question: how do adolescents develop a stable and coherent self-concept in contemporary times? To address the question whether and how self-concept is formed by biological adolescent-specific transitions relative to social influences, we cover literature with a focus on childhood and adolescence that is based on brain imaging, puberty, behavioral and self-report studies, and youth participatory action research (Y-PAR). Notably, none of these approaches individually offers comprehensive answers; however, studies in relation to each other can provide important complementary perspectives on self-concept development in adolescence. Neuroscientific methods, including structural and functional brain imaging, have significantly impacted our understanding of behavioral transitions in adolescence. They offer a more in-depth and detailed analysis of processes that may be challenging to understand solely through experimental tasks or self-report measures (Casey, 2015; Luna et al., 2015). For example, when adolescents are faced with decisions involving immediate versus delayed rewards in the future, a process referred to as delay discounting (Casey et al., 2011; Mischel et al., 1989), experimental tasks typically only provide the final outcome of the decision. However, this decision-making process can be influenced by multiple factors not fully captured in behavioral responses, while neuroimaging measures can provide insight in the underlying processes involved.
Functional magnetic resonance imaging (fMRI) studies demonstrated that at least two brain networks are involved in these decisions, one network often associated with immediate reward processing and one network that is often associated with cognitive control of our actions (McClure et al., 2004; van den Bos et al., 2014). Thereby, the use of brain imaging can provide additional information on how decisions are reached and provides insights into individual differences in these processes (Christakou et al., 2011; van den Bos et al., 2015). Furthermore, self-report measures can provide us with valuable information on how participants evaluate their own thoughts and feelings (Olson et al., 2007; Palenzuela-Luis et al., 2022). However, self-report is also often sensitive to bias, as participants may have a tendency to present more positive views of themselves to the experimenters compared to their social environment in real-life situations (Rodman et al., 2017). As such, complementing self-report research with behavioral and brain imaging studies can provide an additional level of analysis (Romund et al., 2017).
In this review, we will take a complementary approach to demonstrate how to test and refrain developmental theory by using a combination of novel perspectives. First, we will cover the theoretical basis of self-concept development based on Harter’s influential work on the construction of self (Harter, 1999, 2012). We highlight how biological-genetic versus social-environmental influences can be examined through twin models (Achterberg et al., 2018; Harden et al., 2017; van Drunen et al., 2021), serving as a tool for testing developmental theories and how individual differences in self-concept may arise. Second, we present recent neuroscientific models on the construction of self, based on fMRI research that used self-referential processing tasks to examine the neural correlates of self-referential thoughts (Denny et al., 2012). Third, important milestones of structural brain development, including individual differences in genetic and environmental influences, are described providing testable hypotheses regarding self-development in adolescence (Brouwer et al., 2022). Fourth, we unravel the developmental trajectories of self-referential processing across childhood and adolescence and the effects of self-concept training intentions on behavioral and neural outcomes (Crone et al., 2022). In the final section, we discuss how to interpret findings from measures that differ in stability and variability and outline how youth participation studies can provide important clues for relating these scientific findings to the actual experiences of youth in contemporary times (Choudhury et al., 2023).
Models Based on Developmental Theory
Already at a young age, infants develop a sense of self, for example, when babies discover that they have agency on the objects around them. However, it is not until childhood that children start to question who they are in terms of positive and negative traits, and this process becomes more sophisticated in puberty and adolescence (van der Aar et al., 2018). The most influential theory on self-concept development was developed by the seminal work The Construction of Self (Harter, 1999, 2012). Self-concept is defined as the knowledge and beliefs one has about oneself. Developing a positive, realistic, and stable self-concept was previously demonstrated to be predictive for many developmental milestones in life, such as education and job/societal success (Oyserman et al., 2012; Robins et al., 2008; Schwartz et al., 2012). There is a benefit of having a stable self-concept across time, with knowledge and beliefs about ourselves that are clearly defined, also referred to as self-concept clarity (Campbell et al., 2003). However, there is an advantage in the adaptability of self-concept, as it allows to adjust the perception of self in various situations and contexts (Robins et al., 2008). According to Harter’s construction of self-theory, self-concept is a socio-cognitive construct that is shaped by both developmental milestones in cognitive development as well as by social experiences (Harter, 1999, 2012).
Transitions in Self-Concept: Theoretical Perspectives
But how do you measure a complex process such as self-concept? Many theories are based on self-assessments, such as questionnaires that pose individuals with different statements about themselves (Branje et al., 2021; Orth & Robins, 2014). One example is a self-appraisal questionnaire in which individuals respond to traits such as “I am smart” or “I have many friends” (Harter, 1985). Participants are asked to indicate on a scale to what extent these statements apply to them. Harter’s theory describes two important transitions in how children and adolescents evaluate themselves on these self-descriptions that can be used as guiding principles for understanding neural development of self-concept (Harter, 2012) (see Table 1).
Overview of the developmental processes in the forming of self Harter (1988)
| . | Early childhood . | Middle childhood . | Adolescence . |
|---|---|---|---|
| Valence, accuracy | Self-statements extremely positive. Inflated sense of self. Inaccuracies due to confusion between ideal and the real self | More accurate appraisal of self, due to ability to use social comparison and to realistically observe the self. Both positive and negative self-evaluation | Adolescent egocentrism may preclude accurate appraisals of abstractions which define the self and are less testable. Vacillation from positive to negative self-evaluations within the same domain or role |
| Structure content | Specific examples of observable physical characteristics, behaviors, abilities, preferences, possessions | Trait labels reflecting the ability to integrate behaviors into generalized concepts about the self. Focus on abilities, interpersonal characteristics, and emotional attributes, including self-affects | Abstractions about the self, involving psychological constructs, due to the ability to integrate traits into higher order generalizations. Abstractions focus on different roles and relationships |
| Organization | Little coherence to description of self, due to inability to logically organize single self-descriptions | Self-attributes logically organized, integrated within domains which are differentiated from one another | Ability to construct a formal theory of the self in which all attributes across and within role domain are integrated and should be internally consistent |
| Stability, interest | Self-descriptions not stable over time, little constancy, although no recognition, interest, or concern | Recognition of, interest in, continuity and stability of self-attributes over time | Intrapsychic conflict and confusion over contradictions and instability within the self, concern with creation of an integrated identity. Intense preoccupation with the self |
| Bases, criteria | Fantasies, wishes dominate description of behaviors and abilities rather than direct self-observation | Use of social comparison due to ability to simultaneously observe and evaluate the self in relation to others in one’s reference group | Intense focus on the opinions which significant others hold of the self, particularly opinions of peers and close friends |
| Ability to evaluate | Criticism of others, but inability to critically observe and evaluate the self | Aware that others are critically evaluating the self; adopts these attitudes and standards in forming the looking-glass self | Creation of imaginary audience which is critically evaluating the self. Blurs the distinction between this evaluation and self-criticism |
| . | Early childhood . | Middle childhood . | Adolescence . |
|---|---|---|---|
| Valence, accuracy | Self-statements extremely positive. Inflated sense of self. Inaccuracies due to confusion between ideal and the real self | More accurate appraisal of self, due to ability to use social comparison and to realistically observe the self. Both positive and negative self-evaluation | Adolescent egocentrism may preclude accurate appraisals of abstractions which define the self and are less testable. Vacillation from positive to negative self-evaluations within the same domain or role |
| Structure content | Specific examples of observable physical characteristics, behaviors, abilities, preferences, possessions | Trait labels reflecting the ability to integrate behaviors into generalized concepts about the self. Focus on abilities, interpersonal characteristics, and emotional attributes, including self-affects | Abstractions about the self, involving psychological constructs, due to the ability to integrate traits into higher order generalizations. Abstractions focus on different roles and relationships |
| Organization | Little coherence to description of self, due to inability to logically organize single self-descriptions | Self-attributes logically organized, integrated within domains which are differentiated from one another | Ability to construct a formal theory of the self in which all attributes across and within role domain are integrated and should be internally consistent |
| Stability, interest | Self-descriptions not stable over time, little constancy, although no recognition, interest, or concern | Recognition of, interest in, continuity and stability of self-attributes over time | Intrapsychic conflict and confusion over contradictions and instability within the self, concern with creation of an integrated identity. Intense preoccupation with the self |
| Bases, criteria | Fantasies, wishes dominate description of behaviors and abilities rather than direct self-observation | Use of social comparison due to ability to simultaneously observe and evaluate the self in relation to others in one’s reference group | Intense focus on the opinions which significant others hold of the self, particularly opinions of peers and close friends |
| Ability to evaluate | Criticism of others, but inability to critically observe and evaluate the self | Aware that others are critically evaluating the self; adopts these attitudes and standards in forming the looking-glass self | Creation of imaginary audience which is critically evaluating the self. Blurs the distinction between this evaluation and self-criticism |
A first transition that is central in Harter’s work is forming a realistic view of one’s positive and negative traits. Young children often have an over-positive view of themselves, possibly shaped by praise by their parents, and by having a relative narrow view on contexts to which they can compare themselves. For example, children may more often use temporal self-comparisons in which they compare themselves currently with themselves at a younger age, usually resulting in positive self-comparisons (e.g., “I am smarter than I was last year”). Approximately around the onset puberty, adolescents switch from temporal to social comparisons (Pfeifer & Peake, 2012). Their social world becomes larger, and adolescents are more inclined to compare themselves with, for example, their friends and peers (e.g., “I am not as smart as my friend”). Processes that may underlie these developmental transitions may be related to a developmental increase in the capacity for cognitive perspective taking, which results in spontaneously switching perspectives between self and others’ point of view (Dumontheil et al., 2010). A second transition that takes place simultaneously is a stronger social sensitivity to opinions of peers and wanting to fit in (Rodman et al., 2017). Both processes can lead to a temporary dip in how positively adolescents evaluate themselves, which tend to recover in mid-to-late adolescence as they develop a more realistic self-view. As such, they appreciate that perfection in every dimension is not always achievable (van der Aar et al., 2018).
A second transition that is central in Harter’s work is the transition from a general self-concept to multiple domain-specific selves (Harter, 1999, 2012; Harter et al., 1997). During the teenage years, adolescents describe themselves in ways that are more context-dependent. Where young children describe themselves as a “good person,” adolescents may describe themselves as “kind to their best friends” and “cheeky toward their teachers” (Pfeifer & Peake, 2012). Harter’s model characterizes this transition as a progression, evolving from confusion about who is your true self in mid-adolescence to the acceptance that consistency in one’s self-concept is not always feasible until late adolescence (Harter et al., 1997). The domain-specific nature of self-concept raises an important overarching question: can we conceptualize a domain-general self-concept or is the evaluation of oneself distinct in academic or social contexts, implying the existence of multiple selves (Showers & Zeigler-Hill, 2007)? With the transition to 21st century technology, adolescents also wonder whether they are the same person in real-life as online (Konijn et al., 2015). Is the online world simply an additional domain, such as the academic world or the family context, or is it fundamentally different and always present?
Taken together, Harter’s theory suggests that cognitive and social processes are important building blocks for developing a coherent sense of self, resulting in self-concept clarity and self-appraisals across various domains in life (e.g., [pro-]social, academic, and physical). However, this framework can benefit from increased specificity. In this review, we explore whether specificity can be gained by examining recent findings on (1) the prolonged and nonlinear structural brain development during childhood and adolescence, (2) insights from neuroimaging studies using self-concept appraisal paradigms, and (3) self-concept development in the context of 21st century global challenges (i.e., balancing offline and online self, and dealing with societal pressures such as climate change and inequality). Before addressing these theoretically compelling questions, we first discuss one of the leading questions in developmental psychology: how can we unravel whether our thoughts and behaviors are shaped by biology (i.e., genes) or by social experiences (i.e., environment)? We introduce twin modeling as a scientific method to test the relative contributions of genes and environment.
Genetic versus Environmental Influences
When unraveling protracted neural and behavioral developmental time courses, it is often unclear whether these developmental trajectories are determined by late-expressing genetic effects (i.e., biology) or influenced by environmental contributions (i.e., experiences). A well-established method to test these influences is by using twin modeling, which allows for the assessment of genetic (i.e., heritability) and environmental effects (shared environment and unique environment) on brain and behavior. Classical twin designs typically compare monozygotic (MZ) twin pairs, who share 100% of their genes, with dizygotic (DZ) twin pairs, who share approximately 50% of their genes. Two key assumptions underlying these models are that both MZ and DZ twins share a comparable home environment and that twin pairs are of the same sex. As such, two steps of analysis can be incorporated, referred to as within twin-pair correlations and structural equation ACE modeling. As a first step, examining within twin-pair correlations can show that genetic contribution can be informed by a significantly higher correlation in MZ twins compared to DZ twins. In contrast, similarly high within twin-pair correlations in both MZ and DZ twins indicate a shared environmental influence (i.e., the additive effects of a higher proportion of shared genes disappear). As a second step, structural equation ACE modeling can be used to estimate the relative contributions of additive genetic (A), common environmental (C), and unique non-shared environmental/measurement error (E) factors (Neale et al., 2016), based on twin similarities and dissimilarities. Given that MZ twins share 100% of their genes and DZ twins share 50%, the correlations within a twin pair for genetic factors (A) are set to r = 1.0 for MZ twins and r = 0.5 for DZ twins. Additionally, since both MZ and DZ twins share the same home environment, the correlation for shared environmental factors (C) is set to r = 1.0, while the correlation for unique environmental factors (E) is freely estimated. See Figure 1 for an overview of twin model analyses.
Classical twin modeling typically includes two methodology approaches of assessing genetic and environmental effects. First, genetic influences are indicated by a higher within twin-pair correlation in monozygotic (MZ) twins than dizygotic (DZ) twins. High within twin-pair correlations in both MZ and DZ twins are suggestive of shared environmental influences. As a next step, structural equation ACE modeling can offer estimates of the relative contributions that are explained by additive genetic factors (A), common shared environment (C), and unique non-shared environment/measurement error (E).
Classical twin modeling typically includes two methodology approaches of assessing genetic and environmental effects. First, genetic influences are indicated by a higher within twin-pair correlation in monozygotic (MZ) twins than dizygotic (DZ) twins. High within twin-pair correlations in both MZ and DZ twins are suggestive of shared environmental influences. As a next step, structural equation ACE modeling can offer estimates of the relative contributions that are explained by additive genetic factors (A), common shared environment (C), and unique non-shared environment/measurement error (E).
This review aimed to advance our theoretical insights on self-development by testing the effects of genetic and environmental influences and integrating recent knowledge about the structurally and functionally developing human brain based on developmental theory. Before delving into these developmental questions, we first highlight recent discoveries based on understanding the neural processes that are related to self-evaluations in adults.
Neuroscience Models for Thinking about Self
Can a construct as complex as self-concept be examined at the brain level? This question has now been addressed for 2 decades, yielding promising outcomes as well as thoughtful questions for future research. Many neuroscientific models go through stages of discovery (i.e., unraveling active brain networks related to certain cognitive processes), testing (i.e., expecting brain activity in these networks based on experimental tasks), replication (i.e., confirming activation across studies), and prediction (i.e., meaningfully linking activity to task behavior or developmental outcomes) (Rosenberg et al., 2018). These steps have proven useful to advance the field of neuroscience and to ultimately connect with theoretical models.
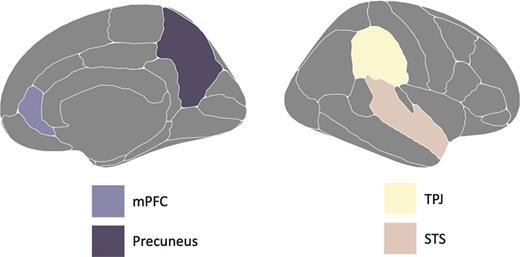
Based on meta-analyses across more than hundreds of studies, researchers have unraveled a brain network referred to as the “social brain” that is consistently active when individuals think about intentions of self and others (Andrews et al., 2021; Van Overwalle, 2009; Van Overwalle et al., 2014). This network involves the medial prefrontal cortex (mPFC), precuneus, temporal parietal junction (TPJ), and superior temporal sulcus (STS). Some regions overlap with what traditionally is referred to as the default mode (DMN) network (Raichle, 2015; Sezer et al., 2022), a network of brain regions that is activated during rest phases of a task, which has been linked to self-referential thought (Davey et al., 2016). See Figure 2 for an overview of the brain regions that are involved in the “social brain” network.
A visualization of the social/self brain network including the medial prefrontal cortex (mPFC), precuneus, temporoparietal junction (TPJ), and superior temporal sulcus (STS).
A visualization of the social/self brain network including the medial prefrontal cortex (mPFC), precuneus, temporoparietal junction (TPJ), and superior temporal sulcus (STS).
Despite that traditionally this network was discovered as the “social brain,” subsequent studies showed that part of this network is also involved in self-related processing. Researchers that were interested in studying neural correlates of self-concept have mostly worked with self-appraisal paradigms, in which participants read short sentences with self-related traits (e.g., “I am athletic”) and rate on a scale the extent to which these individual traits apply to them. These studies showed that evaluating self-traits relative to control items (e.g., counting the number of syllables) was associated with increased activity in the cortical midline regions of the brain, particularly the mPFC and the precuneus (Ochsner et al., 2005). Follow-up studies confirmed in large meta-analyses that the mPFC is consistently activated in self-appraisal paradigms. The identical network is engaged when self-evaluations are conducted from one’s own perspective or from the viewpoint of a reflected other/observer, with distinctions along the dorsal-ventral axes dependent on whether the evaluation concerns personally relevant evaluations (i.e., ventral pathway) or evaluations from the perspective of more distant others (i.e., dorsal pathway) (Denny et al., 2012). These findings suggest that self-evaluations are inherently social; we construct an image of ourselves based on reflected appraisals by others. Finally, across studies, it was observed that evaluating positive traits resulted in stronger activity in the mPFC, especially in the ventral parts, than evaluating negative traits, possibly because positive traits feel more personally relevant (D’Argembeau, 2013). The overlap of regions correlated with social processes (the social brain network) and self-related processes (the self brain network) has been previously interpreted as that the self is intimately intertwined with self-evaluations by others. This integration establishes a central hub region in the brain for processes involving the self and others (Crone & Fuligni, 2020).
While neuroimaging research robustly discovered a key midline brain network (mPFC and precuneus) associated with self-processing, Harter’s theoretical framework suggests that self-concept evaluations can differ between domains. Indeed, recent studies confirmed that the mPFC and precuneus work in close concert with other regions in the brain that are more selectively activated for different domains. In our own work, we specifically compared neural activation associated with self-evaluations in the academic (e.g., “I am smart”), (pro)social (e.g., “I help others”), and physical (e.g., “I have beautiful eyes”) domain (van der Cruijsen et al., 2017; van Drunen et al., 2021). First, when thinking about academic traits, the precuneus in adolescence and adulthood (van der Cruijsen et al., 2017) and dorsolateral prefrontal cortex (DLPFC) in childhood (van Drunen et al., 2021) are relatively more active for academic traits specifically. The precuneus region has previously been associated with autobiographical memories, so possibly evaluating academic traits is more dependent on evaluating yourself in the context of time (Pfeifer & Peake, 2012). In contrast, evaluating traits in the physical domain was strongly associated with the cognitive control areas in the brain, including the anterior cingulate cortex and DLPFC in adolescence (van der Cruijsen et al., 2018) and adulthood (van der Cruijsen et al., 2017). Finally, the (pro)social evaluations were not associated with unique activation above the shared self-referential processing network of mPFC (van der Cruijsen et al., 2017, 2018; van Drunen et al., 2021). Interestingly, the regions that are associated with self-referential processing are overlapping with the DMN, and this network is typically anti-correlated with the cognitive control network in the brain (Raichle, 2015). As such, this leaves open an important question on how individuals switch between thinking about themselves and using cognitive resources that rely on control regions. The precuneus, which is part of both the core self-referential network and the domain-specific academic network, is thought to function as a hub region that connects the DMN to the cognitive control network (Li et al., 2019).
Taken together, neuroscientific research in children, adolescents, and adults has developed promising models in only 20 years of time that have allowed researchers to discover, test, and replicate neural networks that are associated with aspects of self-processing. The key element for testing theoretical models, however, lies in the question of prediction (Rosenberg et al., 2018). Is brain activity meaningfully associated with developmental stage, task performance, and developmental outcomes, currently and in the future? To test these questions, we first will cover recent discoveries on growth trajectories of the structural brain, including assessments of genetic versus environmental influences to better understand how individual differences originate. This will then provide foundational components to confirm and refine developmental theory in relation to important developmental milestones using cross-sectional and longitudinal designs.
Structural Development of the Brain
General Brain Development
During development, the brain undergoes profound changes in various morphometric properties of its structure, including gray and white matter volume, surface area, and cortical thickness (Gogtay et al., 2004; Tamnes et al., 2017; Vijayakumar et al., 2016). Each of these magnetic resonance imaging (MRI) phenotypes shows key developmental milestones during development, reflected in peaks, increases, and decreases (Bethlehem et al., 2022). As such, prior work demonstrated that cortical gray matter volume (i.e., product of cortical thickness and surface area) increases during childhood, peaks in late childhood, and subsequently decreases during the second decade of life before it stabilizes in early adulthood (Aubert-Broche et al., 2013; Mills et al., 2014; Tamnes et al., 2017; Wierenga et al., 2014). Simultaneously, there is a consistent growth in white matter volume throughout childhood and adolescence, peaking at approximately end 20s (Bethlehem et al., 2022). Surface area shows a similar developmental trajectory as gray matter volume, with both measures reaching their highest point around 11–12 years of age. On the other hand, cortical thickness peaks earlier (at approximately 2 years of age), followed by a decrease until adulthood (Bethlehem et al., 2022; Wierenga et al., 2014). Note that there are individual differences in the timing and trajectories of various regions in both intercept (e.g., overall cortical thickness level) and slope (e.g., changes over time) (Foulkes & Blakemore, 2018; Mills et al., 2021) which are at least partly influenced by puberty (Goddings et al., 2014; Vijayakumar et al., 2018). Another important direction in gaining a deeper understanding of variability in structural brain developmental trajectories is by linking it to variability related to genes and environment.
Heritable and Environmental Influences on Brain Development
Areas within the “social brain” network, which reach their peak of volumetric increases in late childhood, are among the latest regions to mature and show protracted growth into adulthood (Mills et al., 2014). However, whether these protracted developmental trajectories are determined by late-expressing genetic effects and/or driven by environmental influences remain to be unraveled. We describe results from our longitudinal Leiden Consortium on Individual Development (L-CID) twin cohort (Crone et al., 2020) which allows for the assessment of heritability and environmental contributions on brain and behavior. Prior L-CID studies showed that brain structure of social regions in 7–9-year-olds was influenced by a combination of genetic and environmental effects (Van der Meulen et al., 2020; van Drunen et al., 2024). More specifically, findings indicated genetic influences on surface area and cortical thickness for all regions of the social brain. Arguably, a genetic blueprint is needed for the foundational structure of the organization of the brain (Fox et al., 2010). This framework could be the basis for processing and responding to the surrounding environment (Hammock & Levitt, 2006) and may function as a building block for the development of a more refined structure driven by additional (social) environmental factors (Fox et al., 2010; Lindenberger & Lövdén, 2019). Indeed, shared environment additionally influenced the TPJ, STS, and precuneus (Van der Meulen et al., 2020; van Drunen et al., 2024). Importantly, in line with the impact of the environment on TPJ and STS during childhood, the developmental changes in these areas also reveal an environmental effect in the same individuals who were followed from 7 to 14 years of age (van Drunen et al., 2024). These findings postulate that TPJ and STS might be relatively most sensitive for social experiences from middle childhood merging into early adolescence. Taken together, these results underscore that variations in structural “social and self” brain development are not exclusively derived from genetic factors. They highlight that the developmental period between childhood and adolescence might be a critical time window during which individuals may be particularly susceptible to (social) environmental/experiential influences.
Social Experiences and Intervention Effects on Brain Development
The environmental influences suggest that the slope of structural brain development in these social regions can be affected by social experiences (Blakemore & Mills, 2014). For example, a prior study showed that a more rapid cortical thinning of mPFC surface area development was indicative of a more pronounced improvement in friendship quality in 8–25-year-olds (Becht et al., 2020). The closest approximation of causal inferences comes from large-scale societal interventions. In this context, we previously examined in a time- and age-controlled longitudinal study whether and how social brain development of teenagers was affected by growing up during COVID-19 pandemic interventions (van Drunen et al., 2023), such as experiencing consequences of social isolation and school closings (Andrews et al., 2020; Orben et al., 2020). Participants in the age range of 11–13-years either participated in two sessions just before the pandemic (pre-pandemic) or in one session before and the other session during the pandemic (peri-pandemic group). Given the variability in ages, it was possible to control for age. This study demonstrated accelerated effects of mPFC thickness development and recovery to negative effects of TPJ surface area development in teenagers who were in the peri-pandemic relative to the pre-pandemic group (van Drunen et al., 2023). Taken together, the developmental period between childhood and adulthood, in which individual differences in pace of brain maturation are taking place, may serve as a window of opportunity for social and self-concept development (Crone et al., 2022; Fuhrmann et al., 2015).
Functional Neural Development of Evaluating Self
Using functional neuroimaging studies including judicious task manipulations, we gain insights into whether self-processes rely on overlapping or separable brain regions and their developmental linear versus nonlinear time courses.
Functional Neural Correlates of Self-Concept in Childhood
The ability to describe oneself in various domains develops in adolescence through increasing social comparisons (Harter, 2012). Therefore, an important question arises how neural representation of self-concept is developed in childhood to serve as a basis for understanding increasingly complex forms of self-concept in adolescence. It is not yet well understood whether and how the social environment influences self-concept forming across distinct domains. We addressed this question in a study of 7–9-year-olds in which we investigated genetic and environmental effects on neural and behavioral aspects of self-concept using a twin design (van Drunen et al., 2021). The findings revealed increased activation in the mPFC during self-related evaluations in comparison to the control condition. This effect was more pronounced for social self-evaluations than for academic self-evaluations. In contrast, heightened activation in the DLPFC was observed for academic evaluations compared to social evaluations. By defining self-concept in two domains (academic and social), the study also revealed domain-specific heritability estimates on both behavioral and neural levels. Behaviorally, we observed shared environmental contributions to social self-concept but genetic contributions to academic self-concept. In line with this behavioral finding, mPFC (and right anterior PFC) activity was influenced by environmental factors for social self-evaluations and by genetic factors for academic self-evaluations. The study confirmed shared environmental influence on the activity of the mPFC during social self-evaluations (aged 7–9 years) depending on the specific domain (van Drunen et al., 2021). These findings complement a prior longitudinal study that showed that changes in mPFC are influenced by pubertal development in the social domain, but not in the academic domain (Pfeifer et al., 2013). Taken together, these findings show that already at a relatively young age (7–9-year-olds), there is domain differentiation at the neural level, partly overlapping with prior results in adults. However, based on developmental theory, we expect that this differentiation becomes more sophisticated during adolescence.
Functional Neural Correlates of Self-Concept in Adolescence
To address the question how self-concept development and domain differentiation take place during adolescence, developmental fMRI studies examined developmental time courses of neural activity during adolescence. Several studies, including longitudinal research, have confirmed nonlinear changes in behavioral self-evaluations in adolescence (Pfeifer et al., 2007, 2013; Somerville et al., 2013; van der Cruijsen et al., 2023). That is, self-evaluations show an initial decrease in positivity from childhood to mid-adolescence, followed by a recovery in late adolescence (van der Cruijsen et al., 2023). This is consistent with the notion of Harter suggesting a temporary dip in how adolescents evaluate themselves (Harter, 1999, 2012). This decline was observed across all domains, yet it was strongest for the academic domain. This observation may indicate that adolescents perceive fewer opportunities to adjust their window of possibilities and possess less agency over immediate outcomes. Consistent with this pattern, functional MRI studies observed that the mPFC is relatively more active in early- to mid-adolescence than in young adults (van der Cruijsen et al., 2023), although some caution is needed because studies in specific age ranges did not confirm this pattern for self-concept development in, for example, 10–13-year-old adolescents (Barendse et al., 2020). The exact age-related pattern is dependent on various factors including, for example, the baseline tasks that are used (Crone et al., 2022). Consistent with the observation that positive traits are more often endorsed than negative traits, positive trait evaluation results in stronger mPFC activity than negative traits (Moran et al., 2006; Van de Groep et al., 2021; van der Cruijsen et al., 2018), possibly reflecting self-relevance or significance (D’Argembeau, 2013). However, this pattern of stronger mPFC activity for positive traits was observed across ages (van der Cruijsen et al., 2018).
The exact functional role of the mPFC in neural responses to self is not yet fully understood (Burnett et al., 2011). The mPFC may support the updating of more domain-general information about the self (Cosme et al., 2021). Having a stable and coherent domain-general knowledge structure of self depends on a constant process of updating and constraining information that is personally relevant and salient in the context of self-knowledge. As such, this general process may be the functional role of the mPFC. However, the mPFC is connected to many other regions in the brain that may contribute to knowledge updating (Zamani et al., 2022). This updating may occur based on the valence of the self-evaluation (Somerville et al., 2013), consistent with Harter’s notion of temporal to social self-comparisons (Harter, 1999, 2012), and for domain-specificity of self-evaluation (van der Cruijsen et al., 2018), consistent with Harter’s notion of contextual differentiation of self-concept (Harter, 1999, 2012).
To test for developmental differences in domain-specific neural recruitment, we rely on few studies that have examined this question in a developmental study (Jankowski et al., 2014; Pfeifer et al., 2009). In our own work, we noticed that the neural differentiation between domains observed in adults (van der Cruijsen et al., 2017) and in children (van Drunen et al., 2021), was also observed in adolescents (van der Cruijsen et al., 2018). That is, adolescents recruited the precuneus more strongly for academic traits and the DLPFC and dorsal medial frontal cortex/anterior cingulate cortex more strongly when evaluating physical traits. The latter was associated with a linear age-related developmental increase in activity, possibly showing that domain-specific activity increases throughout the transition into adolescence (van Drunen et al., 2021). These findings fit within our model, suggesting that protracted development of cognitive control and perspective taking may mediate the relation between age and neural activity, resulting in adolescents developing more complex and differentiated self-concept (Crone et al., 2022).
Training Self-Concept in Adolescence and Emerging Adulthood
The evidence for environmental influences on self-concept development leads to the question whether self-concept can also be trained using interventions. Multiple studies have provided evidence for several forms of self-concept training, awareness, or acceptance (Palenzuela-Luis et al., 2022). However, very few have examined the neural correlates of changes in self-evaluation over time. To test this question in a naturalistic environment, we previously asked 38 participants (16–24-year) who signed up for a gap year program to participate in a longitudinal fMRI study (Van der Aar et al., 2021). Participants came to the laboratory twice, once before and once after the gap year for an fMRI session and completed behavioral tasks midway and 6 months following the gap year program. At baseline, participants were compared with a control group that did not participate in the gap year program. Participants showed the same pattern of neural activity as in prior studies, including more activity in mPFC when evaluating self-traits and more activity for positive than negative traits. However, interestingly, we observed that participants in the gap year program had lower self-esteem than participants who did not participate in the gap year program. Moreover, participants with higher self-esteem showed stronger activity in the mPFC during self-evaluations (van der Aar et al., 2019), possibly indicating more coherent domain-general self-knowledge (Somerville et al., 2010).
The gap year program was targeted to individuals who struggled with academic decision-making, however, had a broader focus beyond academic choices alone. Elements of the program were related to self-knowledge, self-acceptance, and gaining positive social experiences, for example, through volunteer work or traveling. When participants were followed during the duration of the gap year, they showed increases in positive social self-evaluations and in self-esteem across time points. Self-concept clarity also increased over time, yet slower than the increase in self-esteem, possibly suggesting that effects of self-concept training need more time to result in self-concept clarity changes. Increases in self-concept clarity were also associated with better social and academic adjustment at the final time point. At the neural level, we observed an increase in mPFC activity, specifically for the positive trait evaluations (Van der Aar et al., 2021). These findings demonstrate, together with the shared environmental influences in mPFC activity (van Drunen et al., 2021), that the mPFC is sensitive to environmental enrichment.
Stability, Variability, Growth, and Validity
The studies outlined so far suggest that structural brain measures, functional brain measures, and behavioral measures all point in similar directions to explain developmental trajectories of self-concept development. However, these studies did not yet unravel links between these measures at an individual level. Thus, at the group level, the trends are consistent, but an important question is whether and how these results translate to the prediction level (Rosenberg et al., 2018). How can we translate these findings to meaningful theoretical models? To answer these questions, it is worthwhile to have an index of which measures reflect stability, variability, and growth.
Stability is an important index in scientific studies. Stability allows you to find person specific indices, that are stable across time. Structural brain development measures, for example, are highly consistent across time, with intraclass correlations coefficients (ICCs) in the range of 0.90 (Becht et al., 2020). This gives faith that the measure is capturing a real signal. However, note that when stability is too high, it is no longer informative for testing individual differences in developmental change patterns. Measures with high stability can, however, be very informative for understanding individual differences in time-independent variables and can therefore also inform how stable person specific indices (e.g., DNA) interact with variable processes (e.g., behavioral measures) (Ellis et al., 2011).
Variability is also an important index for scientific research, especially in the context of developmental changes. Variability may indicate that states of an individual are changing over time due to environmental circumstances (de Vries et al., 2023), illustrated by prior studies showing that prefrontal cortex activity is sensitive to (spontaneous) variability in mental states (Zamani et al., 2022). Mood, for example, is highly variable between and within individuals and often correlates with other circumstances such as feeling supported by the environment (Bulow et al., 2022). The downside of variability is that it can be difficult to distinguish a real signal from noise. For example, fMRI signals frequently exhibit high variability over time, with ICCs that can be as low as 0.1–0.3 (Braams et al., 2015; Ordaz et al., 2013) (see Fig. 3). Yet, simultaneously, these fluctuating fMRI signals were found to be meaningfully associated with current task performance, sleep, and mood (Flournoy et al., 2024).
Intraclass coefficients (ICCs) of studies from our laboratory described in this review based on the longitudinal Brain Time study (8–28-year-olds) (Blankenstein et al., 2019; Bos et al., 2018; Peper et al., 2018; Schreuders et al., 2018), Leiden Consortium Individual Development (L-CID) study (7–14-year-olds) (Dobbelaar et al., 2023; van der Meulen et al., 2023; van Drunen et al., 2024), and Self-Concept (10–24-year-olds) study (Achterberg et al., 2022; Becht et al., 2018; van der Cruijsen et al., 2023). All studies had at least three measurements and one- to two-year intervals between measurements.
Intraclass coefficients (ICCs) of studies from our laboratory described in this review based on the longitudinal Brain Time study (8–28-year-olds) (Blankenstein et al., 2019; Bos et al., 2018; Peper et al., 2018; Schreuders et al., 2018), Leiden Consortium Individual Development (L-CID) study (7–14-year-olds) (Dobbelaar et al., 2023; van der Meulen et al., 2023; van Drunen et al., 2024), and Self-Concept (10–24-year-olds) study (Achterberg et al., 2022; Becht et al., 2018; van der Cruijsen et al., 2023). All studies had at least three measurements and one- to two-year intervals between measurements.
Growth refers to a combination between stability and variability. Most developmental processes do not develop from one moment to the next but are characterized by stochastic development of variability and change (Pachucki et al., 2015). This may sometimes be mistakenly confused with the idea that measures with high variability are not meaningful for detecting change (Elliott et al., 2020). For some measures, it may not lead to prediction at the individual level, but it can be informative for understanding group-level transitions. As an illustration, measuring the temperature in a country on a monthly basis will result in a combination of high variability and low stability. Some countries may show higher variability than others, making predictions challenging. However, measuring the temperature across multiple countries across longer time periods can signal overall worldwide temperature increases, indicative of climate change. Thus, when interpreting change scores with low and high variability, it is crucial to not too hastily conclude that measures with high variability lack meaningfulness in the understanding of growth (Elliott et al., 2020). Figure 3 shows an illustrative meta-exploration of stability of measurements across multiple time scales based on the longitudinal datasets that are presented in this review. As can be seen in this Figure, the various measures show differences in stability across time scales, across contexts, and even across regions in the brain (see also Flournoy et al., 2024). A challenge and opportunity arise when integrating knowledge from neuroimaging (structural, functional), behavior, and self-report, considering the extent to which these measures are showing meaningful stability, context-dependent variability, and capture noise. Ultimately, this will enable us to unravel, for future theory testing, the degree to which the measures can predict outcomes at the individual level, the group level, and the situational level (Rosenberg et al., 2018).
Finally, in addition to the reliability of measures, the validity of the constructs scientists use is equally important. Many self-report questionnaires that measure self-concept were developed 30–40-years ago with few updates over time (Palenzuela-Luis et al., 2022; Rosenberg, 1986). This does not have to be problematic if these measures capture time-independent developmental processes (Trzesniewski et al., 2003). However, the social world of adolescents is rapidly changing, with new ways of communication (e.g., through social media channels such as Instagram and Snapchat) making the previously presumed “imaginary audience” a daily reality for youth (Burrow & Rainone, 2017; Peters et al., 2021; van der Meulen et al., 2017). In addition to the technological developments, there are many other changes in young people’s lives in the last decade, such as global crises that affect many intertwined systems simultaneously, including the COVID-19 pandemic and growing up with the prospect of climate change (Choudhury et al., 2023). Self-descriptions are also subject to change, with higher reports of gender diversity in recent surveys (Kahn et al., 2024). Gender identity can affect self-concept through various mechanisms, including societal influences, personal alignment with gender identity, developmental factors, and the presence or absence of supportive environments (Baron et al., 2013; Burke, 1989; Steiner et al., 2022; Watson et al., 2021). Experimental designs are typically more adaptive to these types of contexts, for example, by designing tasks such as a Chatroom task (Silk et al., 2012) or a driving task (Chein et al., 2011), whereas questionnaires are usually more strongly grounded in historical context (Palenzuela-Luis et al., 2022). In our recent work, we developed a multidimensional well-being task together with youth in participatory designs demonstrating the importance of including youth perspectives on self-concept and well-being to cover the needs of youth in contemporary times (Green et al., 2023). Y-PAR is a well-known method rooted in action theories that state that active participation of communities that are affected by the research is an essential element of the full research cycle (Dedding et al., 2022; Toenders et al., 2024). Y-PAR involves adolescents as participatory researchers, on a topic that they want to understand and change based on their personal interests (e.g., climate change). This may inform how adolescents value themselves in different domains in their lives. Thus, including youth perspectives has much advantage when aiming to understand the world through the eyes of young people (Jacquez et al., 2013; Ozer & Douglas, 2012; Vaughn et al., 2013).
Conclusions
The goal of this review was to examine whether developmental theory on self-concept development can be enriched or constrained based on neuroscientific findings. Working from the theoretical model of Harter (Harter, 1999, 2012), we tested two developmental transitions: nonlinear developmental patterns of self-evaluations and increasing domain differentiation (Pfeifer & Peake, 2012). The results show that behavioral self-report findings and experimental behavioral findings show parallel patterns with the associated neural correlates. Specifically, the results show that the behavioral age-related dip in academic and social self-evaluations occurs in concert with elevated mPFC activity (Somerville et al., 2013; van der Cruijsen et al., 2023). Furthermore, the neural evidence suggests a domain-general function of the mPFC, yet domain-specific neural activity occurs in various brain networks when traits are associated with academic, social, prosocial, or physical domains (Pfeifer et al., 2009; van der Cruijsen et al., 2018; van Drunen et al., 2021). The developmental patterns can be interpreted in the context of large-scale meta-analytic functional MRI studies based on discovery, test, and replication samples (Denny et al., 2012).
We propose that four conclusions can be drawn that inform developmental theory. First, functional MRI studies demonstrated that the same neural regions are recruited when participants evaluate themselves from their own or from a reflected perspective, which become more aligned with increasing age (Van der Cruijsen et al., 2019) and are possibly driven by puberty, especially for the social domain (Pfeifer et al., 2013). Pubertal development was associated with increased activation in the mPFC which may indicate that changes in social self-evaluations are at least partly linked to biological factors, in addition to peer influences. These results suggest that self and reflected self are intertwined; the evaluation of self is in essence a social construct. Second, neuroscientific findings inform developmental theory by providing directions for underlying mechanistic processes that may drive (i.e., mediate) developmental changes (Crone et al., 2022). For example, the finding that (pro)social self-evaluation processes rely on the domain-general mPFC, whereas academic and physical self-evaluation recruit additional brain networks may indicate that some domains recruit different cognitive resources (e.g., autobiographical memories or perspective taking) than others (Pfeifer & Peake, 2012). Third, neuroscientific findings inform developmental theory by showing that the same behavior can result from different underlying neurocognitive processes at different ages. The finding that academic self-concept is associated with increased activity in DLPFC in 7–9-year-old children (van Drunen et al., 2021), but only the precuneus in adults (van der Cruijsen et al., 2017) may indicate that academic self-evaluations rely on fewer or different cognitive resources across development. Finally, we observed that a naturalistic intervention can boost the neural signal in mPFC for individuals who have low self-esteem and struggle with academic choices (Van der Aar et al., 2021), consistent with twin models that demonstrate shared environmental influences for social and self-related brain regions structurally (Van der Meulen et al., 2020) and functionally (van Drunen et al., 2021).
One of the questions that is often posed is how the integration of neuroscientific methods with developmental theory advanced the field’s understanding of developmental processes. This question, however, creates a false dichotomy because it suggests that neuroscientific methods are a separate knowledge source from developmental theory (Crone & Ridderinkhof, 2011). Even though scientific fields emerge separately, for example, in disciplines such as neuroscience and developmental psychology, the separation of these disciplines is not what drives the frontiers in knowledge. Instead, most of the processes that scientists want to unravel do not fall within the borders of disciplines, rather we should wonder: which scientific question do we aim to answer and which methods are available in our scientific toolbox to help with answering this question?
We suggest two important directions for future research and theory development. First, we showed that through the investigation of both structural and functional brain metrics and by demonstrating diverse contributions to individual differences in brain structure and function, these findings emphasize the complex interplay of genetic and environmental influences on the brain. To fully test, develop, or constrain developmental theory, a combination of the many measures, in our toolbox, ranging from structural, functional, and developmental measures is needed to gain a comprehensive understanding of individual differences in genetic and environmental effects on self-concept development. Some of these measures may be more insightful for describing mechanistic change at the group level and have low levels of explained variance at the individual level. Other measures may be more descriptive and have a higher predictive value at the individual level. This insight describes the need for a potential change in the academic landscape. Some questions that arise may need small-scale discovery studies that can be conducted in single laboratories, and others require large-scale team science studies that have the potential to detect the best levels of prediction at population level (Rahal et al., 2023).
Second, this review was limited to studies that included youth as relatively passive contributors to the research, as research participants in the study. This method is based on the classic empirical design where researchers have the lead in formulating the questions and developing the methods. However, youth are the experts of their own lives, and other methods such as Y-PAR may better capture how contemporary youth think of self-concept (Jacquez et al., 2013). With this method, based on design theory, youth are involved in almost every part of the research cycle, informing the research questions, contributing to the design, and reflecting on the outcomes of the study (Choudhury et al., 2023; Toenders et al., 2024). Developing novel theories may not only rely on novel methods (such as the combination of neuroimaging with self-report) but also novel perspectives (i.e., youth, youth workers) (Green et al., 2023). This method is more important in times when the world is rapidly changing (e.g., the industrial revolution, the technological revolution, and more recently the information revolution, including artificial intelligence) (Arnett et al., 2014).
In conclusion, in this review, we explored how neuroscience can inform theory by testing several compelling questions related to stability versus change of neural, behavioral, and self-report measures and reflect on the meaning of variability and change/growth. We also focused on how theoretical perspectives can and should advance our knowledge on how young individuals can develop a stable self-concept in the contemporary society. We argue for a broad perspective on the unique contribution of difference scientific disciplines, valuing that each answer a different question that together inform theories on self-development in meaningful ways. Understanding the interpretation of these findings should include youth as experts of their own lives, such that theoretical advancements are not only based on novel methods but also novel perspectives.
Acknowledgment
The authors thank Ann Hogenhuis for proofreading the manuscript.
Statement of Ethics
An ethics statement was not required for this study type since no human or animal subjects or materials were used.
Conflict of Interest Statement
The authors report no conflict of interest.
Funding Sources
The first author received a grant from the Netherlands Institute for Advanced Study in the Humanities and Social Sciences (NIAS). The first and second authors were supported by a Gravitation program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO Grant No. 024.001.003).
Author Contributions
E.A.C. and L.v.D. contributed equally to writing and conceptualization.
Data Availability Statement
All data presented in this review have been published in prior articles.