Peptide hormones exert unique actions via specific G protein–coupled receptors; however, the therapeutic potential of regulatory peptides is frequently compromised by rapid enzymatic inactivation and clearance from the circulation. In contrast, recombinant or covalent coupling of smaller peptides to serum albumin represents an emerging strategy for extending the circulating t1/2 of the target peptide. However, whether larger peptide-albumin derivatives will exhibit the full spectrum of biological activities encompassed by the native peptide remains to be demonstrated. We report that Albugon, a human glucagon-like peptide (GLP)-1–albumin recombinant protein, activates GLP-1 receptor (GLP-1R)-dependent cAMP formation in BHK-GLP-1R cells, albeit with a reduced half-maximal concentration (EC50) (0.2 vs. 20 nmol/l) relative to the GLP-1R agonist exendin-4. Albugon decreased glycemic excursion and stimulated insulin secretion in wild-type but not GLP-1R−/− mice and reduced food intake after both intracerebroventricular and intraperitoneal administration. Moreover, intraperitoneal injection of Albugon inhibited gastric emptying and activated c-FOS expression in the area postrema, the nucleus of the solitary tract, the central nucleus of the amygdala, the parabrachial, and the paraventricular nuclei. These findings illustrate that peripheral administration of a larger peptide-albumin recombinant protein mimics GLP-1R–dependent activation of central and peripheral pathways regulating energy intake and glucose homeostasis in vivo.
Glucagon-like peptide (GLP)-1 is a 30–amino acid peptide hormone secreted from gut endocrine cells in response to nutrient ingestion that promotes nutrient assimilation through regulation of gastrointestinal motility and islet hormone secretion (1). Infusion of GLP-1 into normal or diabetic human subjects stimulates insulin and inhibits glucagon secretion, thereby indirectly modulating peripheral glucose uptake and control of hepatic glucose production (2). GLP-1 also produces anorectic effects, and short-term infusion of GLP-1 is associated with diminished appetite and reduced energy consumption in normal, obese, and diabetic human subjects (3). Taken together, the actions of GLP-1 to reduce glycemia while preventing concomitant weight gain have attracted considerable interest in pharmaceutical approaches to enhancing GLP-1 action for the treatment of type 2 diabetes.
A major challenge for the therapeutic use of regulatory peptides, including native GLP-1, is a short circulating t1/2, due principally to rapid enzymatic inactivation and/or renal clearance. Although infusion of native GLP-1 is highly effective in lowering blood glucose in subjects with type 2 diabetes, a single subcutaneous injection of the native peptide is quickly degraded and disappears from the circulation within minutes (4). Hence, the majority of pharmaceutical approaches to the development of GLP-1 mimetic agents have focused on the development of long-acting degradation-resistant peptides (5). The naturally occurring lizard salivary gland peptide exendin-4 (Ex-4) is a potent GLP-1 receptor (GLP-1R) agonist and exhibits therapeutic efficacy in studies of patients with type 2 diabetes (6). Similarly, a fatty acylated human GLP-1 analog, liraglutide, exhibits a more sustained duration of action and potently reduces glycemic excursion in diabetic subjects (7).
Although degradation-resistant GLP-1R agonists appear to be promising agents for the treatment of diabetes, the need for once- or twice-daily injection of these peptides has fostered complementary efforts directed at identification of more potent longer-acting agents with sustained efficacy in vivo. Given the long circulating t1/2 of albumin-linked drugs (8), a GLP-1–albumin protein should exhibit a much more prolonged circulating t1/2 and hence requires a reduced frequency of parenteral administration, relative to native GLP-1. Nevertheless, the molecular interaction with the GLP-1R, volume of distribution, and access to the central nervous system (CNS) would be predicted to be markedly different for a much larger albumin-based molecule (8).
Whether all of the desirable actions of native GLP-1, including the activation of the CNS centers regulating food intake and gastrointestinal motility, would be mimicked by a much larger GLP-1–albumin protein is currently unclear. Accordingly, we have examined the biological actions of Albugon, a recombinant GLP-1–human serum albumin (HSA) fusion protein, using a combination of cell line studies in vitro and both wild-type and GLP-1R−/− mice in vivo.
RESEARCH DESIGN AND METHODS
Reagents.
Tissue culture medium, serum, and G418 were purchased from Invitrogen (San Diego, CA). Forskolin and 3-isobutyl-1-methylxanthine were obtained from Sigma (St. Louis, MO). HSA and Albugon were provided by Human Genome Sciences (Rockville, MD). Ex-4 and exendin (9-39) were purchased from California Peptide Research (Napa, CA).
Measurement of cAMP.
Baby hamster kidney (BHK) cells stably transfected with rat GLP-1R were generated and propagated in medium containing 0.05 mg/ml G418 as described previously (9). Before analysis, BHK-GLP-1R cells were grown to 70–80% confluence in 24-well plates in the absence of G418 at 37°C. Ex-4 and exendin (9-39) were dissolved in PBS, and cells were treated with control or test reagents in Dulbecco’s modified Eagle’s medium containing serum and 100 μmol/l 3-isobutyl-1-methylxanthine. Cells were incubated with 1 μmol/l exendin (9-39) or medium alone for 5 min at 37°C, followed by an additional 10-min incubation in the presence of increasing concentrations of Ex-4, HSA, or Albugon. All reactions were carried out in triplicate and terminated by the addition of ice-cold absolute ethanol. Cell extracts were collected and stored at −80°C until assayed. For cAMP determinations, aliquots of ethanol extracts were lyophilized, and cAMP levels were measured using a cAMP radioimmunoassay kit (Biomedical Technologies, Stoughton, MA).
Mice.
All animal experiments were carried out in accordance with protocols and guidelines approved by the Toronto General Hospital Animal Care Committee. GLP-1R−/− mice on the C57BL/6 genetic background, and age-matched (8- to 15-week-old male) wild-type C57BL/6 mice (Charles River Laboratories, Montreal, PQ) were maintained on a 12-h light/dark cycle and allowed free access to standard rodent food and water, except where noted. Wild-type mice were acclimated to the animal facility for a minimum of 1 week before analysis.
Glucose tolerance tests and measurement of plasma insulin.
Oral or intraperitoneal (IP) glucose tolerance tests were carried out after an overnight fast (16–18 h). Glucose (1.5 mg/g body wt) was administered orally through a gavage tube or by injection into the peritoneal cavity. A blood sample was drawn from the tail vein at 0, 10, 20, 30, 60, 90, and 120 min after glucose administration, and blood glucose levels were measured using a Glucometer Elite blood glucose meter (Bayer, Toronto, Ontario, Canada). For plasma insulin determination, a blood sample (100 μl) was removed from the tail vein during the 10- to 20-min time period after glucose administration and immediately mixed with a 10% volume of a chilled solution containing 5,000 KIU/ml Trasylol (Bayer), 32 mmol/l EDTA, and 0.1 nmol/l Diprotin A (Sigma). Plasma was separated by centrifugation at 4°C and stored at −80°C until assayed. Plasma insulin levels were measured using a rat insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Chicago, IL) with mouse insulin as standard.
Measurement of plasma glucagon.
After an overnight fast (16 h), mice were given an IP injection of 1 mg/kg HSA or Albugon. At 20 min after HSA or Albugon administration, mice were killed and cardiac blood was obtained and mixed with a 10% volume of a chilled solution containing 5,000 KIU/ml Trasylol, 32 mmol/l EDTA, and 0.1 nmol/l Diprotin A. Plasma was separated by centrifugation at 4°C and stored at −80°C until assayed. Plasma glucagon levels were measured using a glucagon radioimmunoassay kit (Linco Research, St. Charles, MO).
Feeding studies.
For analysis of food intake, mice were fasted overnight (16–18 h), and control (PBS or HSA) or test (Ex-4 or Albugon) reagents were administered by intracerebroventricular (ICV) or IP injection. For ICV injections, mice were lightly anesthetized using isoflurane inhalation (Abbott Laboratories, Saint-Laurent, Quebec, Canada), and reagents were administered in a total volume of 5 μl by injection into the lateral ventricles using a 2.5 mm × 30-gauge needle attached to a Hamilton syringe as described (10). Mice were allowed to recover from the anesthetic (∼10 min) before assessment of food intake. For IP injections, 100 μl control or test reagent was injected into the peritoneal cavity. Immediately after ICV or IP injection, mice were weighed and then placed into individual cages containing preweighed rodent food, with free access to water. At 2, 4, 7, and 24 h after reagent administration, the food was reweighed and total food intake (g/g of body wt) was calculated.
Gastric emptying.
The gastric emptying rate was determined as described (11). Briefly, mice were fasted overnight (18 h) and then allowed free access to preweighed rodent food for 1 h. After the 1-h refeeding, the remaining food was weighed and food intake was determined. Mice were then given IP injections of PBS, Ex-4 (0.17 mg/kg), HSA (2.7 mg/kg), Albugon (3 mg/kg), or cholecystokinin (CCK)-8 (4 μg) and deprived of food for an additional 4 h. Mice were anesthetized with Somnotol (sodium pentobarbital solution; MTC Pharmaceuticals, Cambridge, Ontario, Canada), their stomachs were removed, and stomach content wet weight was determined. Gastric emptying rate was calculated using the following: gastric emptying rate (%) = [1 − (stomach content wet weight/food intake)] × 100.
Measurement of c-FOS activation in the murine CNS.
The number of c-FOS immunoreactive neurons in specific brain regions was assessed quantitatively in both wild-type C57BL/6 and GLP-1R−/− mice as described (12,13). Briefly, animals were given IP injections of PBS, Ex-4, HSA, or Albugon in a 100 μl volume. At 10 and 60 min after injection, mice were anesthetized with Somnotol. All mice were perfused intracardially with ice-cold normal saline followed by 4% paraformaldehyde solution. Brains were removed immediately at the end of perfusion, kept in ice-cold 4% paraformaldehyde solution for 3 days, and then transferred to a solution containing paraformaldehyde and 10% sucrose for 12 h. Brains were cut into 25-μm sections using a Leica SM2000R sliding microtome (Leica Microsystems, Richmond Hill, Ontario, Canada) and stored at −20°C in a cold cryoprotecting solution. Sections were processed for immunocytochemical detection of FOS using a conventional avidin-biotin-immunoperoxidase method (Vectastain ABC Elite Kit; Vector Laboratories, Burlingame, CA) as described (12). The FOS antibody (Sigma-Aldrich, Oakville, Ontario, Canada) was used at a 1:50,000 dilution. Brain sections corresponding to the level of the area postrema, the nucleus of the solitary tract (NTS), the central nucleus of the amygdala, and the parabrachial and paraventricular nuclei were defined according to the mouse brain atlas of Franklin and Paxinos (14) and selected for analyses.
Statistical analysis.
All data are presented as means ± SE. Statistical significance was determined by ANOVA and Bonferroni post-test using Prism version 3.03 software (GraphPad Software, San Diego, CA). A P value <0.05 was considered to be statistically significant.
RESULTS
Albugon is a recombinant human protein that contains a dipeptidyl peptidase-IV (DPP-IV)-resistant human GLP-1 analog encoded in the same open reading frame as the HSA amino acid sequence. To evaluate whether the bioactive domain(s) of a much smaller peptide hormone could still recognize and functionally interact with its cognate receptor when constrained within a much larger heterologous protein, we examined whether Albugon activated GLP-1R in vitro. Albugon produced a dose-dependent stimulation of cAMP accumulation in BHK-GLP-1R cells that was significantly diminished by coincubation with the GLP-1R antagonist exendin (9-39) (Fig. 1). Nevertheless, Albugon was not as effective in activating the GLP-1R when compared with the much smaller more potent lizard-derived GLP-1R agonist Ex-4 (Fig. 1; EC50 = 0.2 vs. 20 nmol/l for Ex-4 vs. Albugon, respectively).
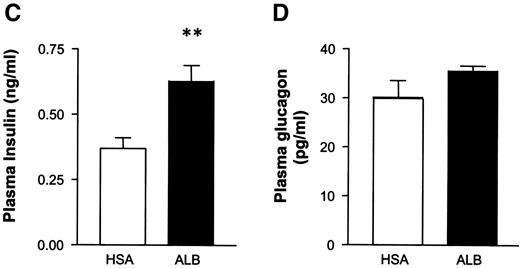
These experiments demonstrate that the considerably altered peptide conformation that arises after insertion of GLP-1 sequences into a much larger albumin open reading frame does not eliminate the ability of essential GLP-1 motif(s) to recognize and activate GLP-1Rs. To ascertain whether circulating Albugon is capable of reaching key sites and activating GLP-1R–dependent actions in vivo, we carried out oral glucose tolerance testing in mice. Glycemic excursion was significantly reduced after parenteral Albugon administration to wild-type mice (Fig. 2A). Furthermore, the glucose-lowering properties of Albugon depended on a functional GLP-1 receptor because Albugon had no effect on blood glucose in GLP-1R−/− mice (Fig. 2B). Consistent with the known actions of smaller GLP-1R peptide agonists, Albugon, at doses ranging from 0.1 to 10 mg/kg, markedly reduced glycemic excursion after not only oral but also IP glucose loading, in association with a significant increase in the insulin-to-glucose ratios (Fig. 3A and B). Albugon increased the levels of plasma insulin after an IP glucose challenge (Fig. 3C) but did not significantly reduce the levels of plasma glucagon in mice after an overnight fast (Fig. 3D). Taken together, these results demonstrate that Albugon lowers blood glucose and enhances insulin secretion through GLP-1R–dependent mechanisms in vivo.
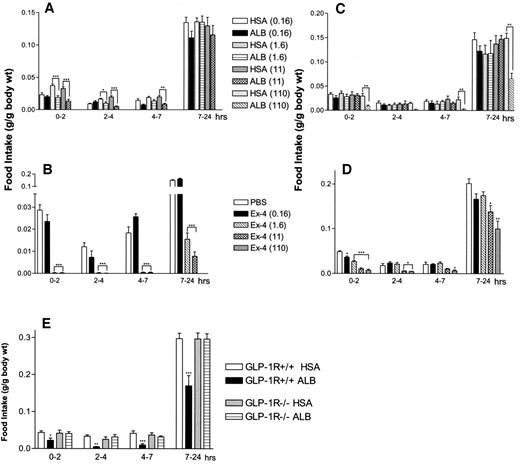
Smaller 30–to 40–amino acid GLP-1R agonists, including native GLP-1, Ex-4, and liraglutide, have been shown to inhibit food intake, presumably via activation of central hypothalamic centers regulating satiety (15). To assess whether a much larger recombinant GLP-1–albumin protein would also exert anorectic effects, we studied food intake in normal mice after ICV or IP peptide administration. ICV Albugon significantly lowered food intake in mice (relative to the HSA control) in a dose-dependent manner, and this effect was detectable for up to 7 h (Fig. 4A) but was not sustained over the entire 24-h observation period. In contrast, Ex-4 exerted a more potent and sustained anorectic effect after ICV administration, with a highly significant reduction of food intake observed even at the end of the 24-h study period (Fig. 3).
The blood-brain barrier is relatively impermeable to larger proteins such as albumin under both normal physiological conditions and in the setting of diabetes (16,17). Although GLP-1Rs are expressed on hypothalamic neurons regulating satiety (18,19), whether GLP-1R agonists require direct access to the brain for activation of the central anorexic pathway remains uncertain (20). Accordingly, we examined whether Albugon would also exhibit anorectic effects after peripheral administration. Although lower doses of IP Albugon, 0.16–11 nmol/kg, did not affect food intake, the highest dose tested (110 nmol/kg) reduced food intake at all time points studied, from 2 to 24 h after Albugon administration (Fig. 4C). In contrast, much smaller doses of Ex-4 significantly reduced food ingestion after IP administration in the same experiments (Fig. 4D). Hence, although Albugon is capable of exerting anorectic actions after both central and peripheral administration, it is less potent compared with the smaller GLP-1R agonist, Ex-4. To ascertain whether the anorectic actions of peripherally administered Albugon were due to the nonspecific effects of the larger amount of injected protein, we repeated the experiments using age- and sex-matched wild-type control and GLP-1R−/− mice. Albugon reduced food intake at all time points in wild-type mice but had no effect on food intake in GLP-1R−/− mice. These findings demonstrate that IP Albugon inhibits food intake in a GLP-1R–dependent manner.
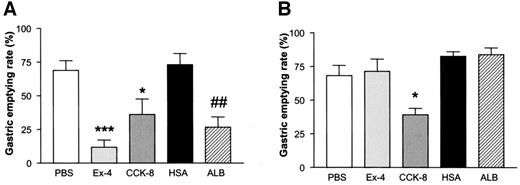
GLP-1R agonists markedly inhibit gastric emptying (21), and gastric distension induces c-FOS in GLP-1–expressing neurons in the rat medulla (22). However, whether direct CNS access is required for either GLP-1R–dependent inhibition of food intake or gastric emptying has not been determined. The basal rate of gastric emptying was comparable in wild-type and GLP-1R−/− mice (Fig. 5). Both Ex-4 and CCK-8 significantly inhibited gastric emptying in wild-type mice; however, CCK-8, but not Ex-4, also inhibited gastric emptying in GLP-1R−/− mice. Similarly, Albugon potently inhibited gastric emptying in wild-type but not in GLP-1R−/− mice (Fig. 5).
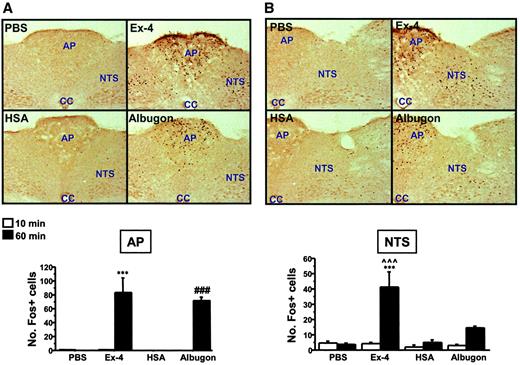
The anorectic actions of small peptide GLP-1R agonists are associated with c-FOS activation in CNS centers coupled with the control of energy intake (18,20,23). To determine whether peripherally administered Albugon was capable of activating neuronal FOS expression, we examined the pattern and extent of CNS FOS expression after IP Albugon administration. Ex-4 markedly increased c-FOS expression in the area postrema, the NTS, the central nucleus of the amygdala, the parabrachial nucleus, and the hypothalamic paraventricular nuclei (Fig. 6A–E). Similarly, Albugon significantly activated c-FOS expression in the identical brain regions, although much less robustly in the NTS and paraventricular nucleus than in Ex-4 (Fig. 6A–E). Analysis of the murine CNS after peripheral (IP) administration of HSA did not detect immunoreactive HSA in brain parenchyma (data not shown), consistent with the inability of HSA to rapidly cross the normal blood-brain barrier (8).
DISCUSSION
GLP-1–based therapies for type 2 diabetes are attracting increasing attention in part because of the preliminary efficacy demonstrated in clinical studies (6) and because of unique yet complementary mechanisms of action (1). Unlike some antidiabetic agents that reduce blood glucose while promoting weight retention, GLP-1R activation is coupled with short-term inhibition of food intake in both rodent and human studies (15). Moreover, prolonged GLP-1 administration for 6 weeks to diabetic human subjects was associated with a significant reduction in body weight over the study period (24). The anorectic properties of GLP-1 and its peptide analogs are thought to be due in part to both inhibition of gastric emptying and activation of central satiety centers coupled with reduction of energy intake (20,25). Although development of GLP-1–based small peptide analogs resistant to enzymatic inactivation is a major focus of current pharmaceutical activity (1), the need for once- or twice-daily injection of these peptides has stimulated efforts toward development of even longer-acting molecules that retain the ability to activate GLP-1Rs.
The relatively prolonged circulating t1/2 of endogenous and exogenously administered albumin has fostered attempts directed at coupling peptide moieties to albumin, thereby increasing the circulating t1/2 of the albumin-peptide complex (26). Albuferon is an interferon-α–albumin fusion protein that retains the biological activity of interferon yet exhibits a markedly extended pharmacokinetic profile relative to native interferon in Cynomolgus monkeys (27). Similarly, fusion of the amino acid sequence of human growth hormone to albumin prolonged the circulating t1/2 of Albutropin relative to the native growth hormone, yet Albutropin retained the ability to stimulate IGF-I and body weight gain in vivo (28). Moreover, peptide binding to albumin extended the pharmacokinetic properties of several smaller proteins including insulin (29), Fab antibody fragments (26), and coagulation factor VIIa inhibitor 1a (30). In contrast to Albugon, however, these albumin-peptide derivatives exert their predominant actions outside the CNS, and activation of the CNS is not critical for their therapeutic actions.
More recent efforts have been directed at extending the t1/2 of the much smaller GLP-1 molecule using albumin-based approaches. Liraglutide is a fatty acylated human DPP-IV–resistant GLP-1 analog that binds to albumin and exhibits a t1/2 of ∼11–15 h after parenteral administration in humans (31). Liraglutide inhibits food ingestion in rats (32) and decreases gastric emptying in human subjects (33); hence, the transient GLP-1–albumin interaction does not preclude communication with CNS centers important for control of satiety and gastrointestinal motility. However, liraglutide binds to albumin in a noncovalent dissociable manner, with the actions of liraglutide attributed to a free peptide unconstrained by its intermittent association with albumin in the circulation.
We recently studied the properties of CJC-1131, a DPP-IV–resistant human GLP-1 analog that covalently couples with HSA after parenteral administration (34). CJC-1131 binds to the GLP-1R and activates a broad spectrum of GLP-1R–dependent actions associated with glucose reduction in db/db mice, including stimulation of insulin secretion and insulin gene expression and expansion of islet mass (34). Intriguingly, CJC-1131 also activates FOS expression in hypothalamic neurons and reduces food intake and weight gain in normal and diabetic mice (23,34). Nevertheless, because CJC-1131 is administered parenterally as the free GLP-1 analog, which subsequently forms a covalent linkage with albumin in vivo (34), it remains likely that some or all of the acute effects of CJC-1131 on the brain reflect the rapid initial actions of free CJC-1131 before covalent coupling with albumin.
In contrast, Albugon contains the sequences of human GLP-1 linked in the same open reading frame with recombinant HSA; hence, no “free” GLP-1 is associated with Albugon administration in vivo. Consistent with studies of CJC-1131 covalently conjugated to albumin (34), Albugon activates the cloned GLP-1R, but with a reduced affinity relative to the potent GLP-1R agonist Ex-4. Because the blood-brain barrier is relatively impermeable to albumin, peripheral administration of the much larger Albugon protein provides an opportunity to determine the relative importance of peripheral versus central GLP-1R networks for control of satiety and gut motility. Remarkably, Albugon inhibited food intake after not only ICV but also after IP administration in mice. Similarly, Albugon significantly inhibited gastric emptying after IP administration. Furthermore, the distribution of neuronal c-FOS activation in different CNS nuclei after peripheral Albugon administration was highly similar, albeit less robust, compared with the pattern of FOS activation observed after Ex-4. These findings support a model whereby peripheral activation of GLP-1R–dependent vagal afferents is capable of activating CNS centers, transducing the effects of GLP-1 in the brain (35,36)
Given the clinical importance of the anorectic actions of GLP-1R agonists for prevention of weight gain in the treatment of diabetes, the mechanisms and pathways activated by GLP-1R agonists that converge on inhibition of feeding centers are of considerable interest. Both native GLP-1 and Ex-4 cross the blood-brain barrier through a GLP-1R–independent pathway (37–39); hence, these peptides are capable of directly penetrating and activating CNS centers after exogenous administration. In contrast, peripheral administration of much larger albumin-based GLP-1R agonists, such as CJC-1131 and Albugon, at doses that do not induce hypoglycemia, rapidly activates neuronal c-FOS in distinct brain regions. Furthermore, intravenous but not ICV CJC-1131 induced tyrosine hydroxylase gene transcription in the area postrema in vivo (23). Hence, our data suggest that peripheral activation of central GLP-1R systems coupled with regulation of FOS expression, gastric emptying, and food intake does not require direct exposure to GLP-1R agonists in the CNS, but may be achieved through activation of ascending pathways coupled with central GLP-1R–dependent networks.
The current data demonstrate that as little as 0.1 mg/kg (1.4 nmol/kg) of Albugon was sufficient for lowering of blood glucose; however, much larger doses (110 nmol/kg), were required for inhibition of food intake after IP administration in mice. These findings imply that acute peripheral Albugon administration is significantly more effective at reduction of glycemic excursion relative to inhibition of appetite. Nevertheless, the experimental design used here did not examine the anorectic effects of more prolonged sustained administration, a paradigm more representative of the potential therapeutic use of Albugon in humans. Furthermore, the t1/2 of circulating HSA, and by inference Albugon, is much longer in humans than in mice (8). Given the intense interest in the development of long-acting GLP-1R agonists for the treatment of type 2 diabetes, the biological properties and mechanisms of action of GLP-1–albumin derivatives merit further investigation.
Albugon exhibits similar efficacy but lower potency than Ex-4 at the rat GLP-1R in vitro. A stable BHK cell line expressing the rat GLP-1R was pretreated for 5 min with 1 μmol/l exendin (9-39) [Ex(9-39)] or medium alone before a 10-min treatment with increasing concentrations of Ex-4, Albugon, or HSA. cAMP levels in lyophilized aliquots of cell extracts were measured by radioimmunoassay and used to calculate total cAMP content per well. Values are expressed as means ± SE and are representative of data from two independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 for Ex-4–vs. Albugon-treated cells.
Albugon exhibits similar efficacy but lower potency than Ex-4 at the rat GLP-1R in vitro. A stable BHK cell line expressing the rat GLP-1R was pretreated for 5 min with 1 μmol/l exendin (9-39) [Ex(9-39)] or medium alone before a 10-min treatment with increasing concentrations of Ex-4, Albugon, or HSA. cAMP levels in lyophilized aliquots of cell extracts were measured by radioimmunoassay and used to calculate total cAMP content per well. Values are expressed as means ± SE and are representative of data from two independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 for Ex-4–vs. Albugon-treated cells.
Albugon lowers blood glucose in wild-type but not in GLP-1R−/− mice. Oral glucose tolerance in wild-type GLP-1R+/+ (A) and GLP-1R−/− (B) mice given IP injections of 5 mg/kg of HSA (○) or Albugon (ALB; ▪) 60 min before an oral glucose load. Values are expressed as means ± SE; n = 5–6 mice/group. **P < 0.01, ***P < 0.001 for Albugon- vs. HSA-treated mice.
Albugon lowers blood glucose in wild-type but not in GLP-1R−/− mice. Oral glucose tolerance in wild-type GLP-1R+/+ (A) and GLP-1R−/− (B) mice given IP injections of 5 mg/kg of HSA (○) or Albugon (ALB; ▪) 60 min before an oral glucose load. Values are expressed as means ± SE; n = 5–6 mice/group. **P < 0.01, ***P < 0.001 for Albugon- vs. HSA-treated mice.
Albugon dose-dependently lowers the glucose excursion and increases plasma insulin and insulin-to-glucose ratios after an oral or peripheral glucose challenge. Oral glucose tolerance test (A) and IP glucose tolerance test (B) in wild-type mice treated with different doses of IP HSA or Albugon (ALB) 60 min before a glucose load. The bottom panel in A and B indicates the plasma insulin-to-glucose ratios (ng/ml:mmol/l) at the 20-min time point after glucose administration in HSA- or ALB-treated mice. C: Plasma insulin levels at the 20-min time point after IP glucose administration in mice treated with 1 mg/kg HSA (□) or ALB (▪). D: Plasma glucagon levels in fasted mice at 20 min after IP administration of 1 mg/kg HSA (□) or ALB (▪). Values are expressed as means ± SE; n = 5–12 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001 for Albugon- vs. HSA-treated mice.
Albugon dose-dependently lowers the glucose excursion and increases plasma insulin and insulin-to-glucose ratios after an oral or peripheral glucose challenge. Oral glucose tolerance test (A) and IP glucose tolerance test (B) in wild-type mice treated with different doses of IP HSA or Albugon (ALB) 60 min before a glucose load. The bottom panel in A and B indicates the plasma insulin-to-glucose ratios (ng/ml:mmol/l) at the 20-min time point after glucose administration in HSA- or ALB-treated mice. C: Plasma insulin levels at the 20-min time point after IP glucose administration in mice treated with 1 mg/kg HSA (□) or ALB (▪). D: Plasma glucagon levels in fasted mice at 20 min after IP administration of 1 mg/kg HSA (□) or ALB (▪). Values are expressed as means ± SE; n = 5–12 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001 for Albugon- vs. HSA-treated mice.
Albugon (ALB) reduces food intake in fasted mice but is less anorectic than Ex-4. After an overnight fast, wild-type mice were given ICV (A and B) or IP (C–E) injections of PBS or increasing doses (nmol/kg) of Ex-4, HSA, or ALB. E: Wild-type and GLP-1R−/− mice were fasted overnight and then given IP injections of 110 nmol/kg of HSA or ALB. Food intake was measured at 2, 4, 7, and 24 h after recovery from injection. Values are expressed as means ± SE; n = 4–5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control-treated (PBS or HSA) mice.
Albugon (ALB) reduces food intake in fasted mice but is less anorectic than Ex-4. After an overnight fast, wild-type mice were given ICV (A and B) or IP (C–E) injections of PBS or increasing doses (nmol/kg) of Ex-4, HSA, or ALB. E: Wild-type and GLP-1R−/− mice were fasted overnight and then given IP injections of 110 nmol/kg of HSA or ALB. Food intake was measured at 2, 4, 7, and 24 h after recovery from injection. Values are expressed as means ± SE; n = 4–5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control-treated (PBS or HSA) mice.
Albugon (ALB) reduces the gastric emptying rate in wild-type but not GLP-1R−/− mice. Gastric emptying rate in wild-type (WT) (A) and GLP-1R−/− (B) mice at 4 h after IP administration of PBS, Ex-4 (0.17 mg/kg), HSA (2.7 mg/kg), ALB (3 mg/kg), or CCK octapeptide (CCK-8; 4 μg/mouse) is shown. Values are expressed as means ± SE; n = 3–4 mice/group. *P < 0.05, ***P < 0.001 vs. PBS; ##P < 0.01 vs. HSA.
Albugon (ALB) reduces the gastric emptying rate in wild-type but not GLP-1R−/− mice. Gastric emptying rate in wild-type (WT) (A) and GLP-1R−/− (B) mice at 4 h after IP administration of PBS, Ex-4 (0.17 mg/kg), HSA (2.7 mg/kg), ALB (3 mg/kg), or CCK octapeptide (CCK-8; 4 μg/mouse) is shown. Values are expressed as means ± SE; n = 3–4 mice/group. *P < 0.05, ***P < 0.001 vs. PBS; ##P < 0.01 vs. HSA.
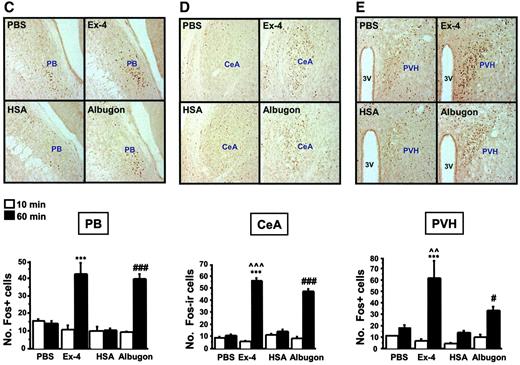
IP Albugon and Ex-4 increase c-FOS levels in the mouse CNS. Representative photomicrographs are shown of c-FOS–stained coronal brain sections of area postrema (AP) (A), NTS (B), hypothalamic parabrachial nucleus (PB) (C), central nucleus of the amygdala (CeA) (D), and paraventricular nucleus of the hypothalamus (PVH) (E) from wild-type mice at 60 min after IP injection of PBS, Ex-4 (11 nmol/kg), HSA (110 nmol/kg), or Albugon (110 nmol/kg). No hypoglycemia was detected after administration of either Albugon or Ex-4 in these experiments. Original magnification, ×200. CC, central canal; 3V, third ventricle. The number of c-FOS immunopositive (Fos+) cells are depicted below the corresponding CNS section. Data are presented as means ± SE; n = 3 mice/treatment. ***P < 0.001 for Ex-4–vs. PBS-treated mice at 60 min; #P < 0.05, ###P < 0.001 for Albugon- vs. HSA-treated mice at 60 min; ∧∧P < 0.01, ∧∧∧P < 0.001 for Ex-4–vs. Albugon-treated mice at 60 min.
IP Albugon and Ex-4 increase c-FOS levels in the mouse CNS. Representative photomicrographs are shown of c-FOS–stained coronal brain sections of area postrema (AP) (A), NTS (B), hypothalamic parabrachial nucleus (PB) (C), central nucleus of the amygdala (CeA) (D), and paraventricular nucleus of the hypothalamus (PVH) (E) from wild-type mice at 60 min after IP injection of PBS, Ex-4 (11 nmol/kg), HSA (110 nmol/kg), or Albugon (110 nmol/kg). No hypoglycemia was detected after administration of either Albugon or Ex-4 in these experiments. Original magnification, ×200. CC, central canal; 3V, third ventricle. The number of c-FOS immunopositive (Fos+) cells are depicted below the corresponding CNS section. Data are presented as means ± SE; n = 3 mice/treatment. ***P < 0.001 for Ex-4–vs. PBS-treated mice at 60 min; #P < 0.05, ###P < 0.001 for Albugon- vs. HSA-treated mice at 60 min; ∧∧P < 0.01, ∧∧∧P < 0.001 for Ex-4–vs. Albugon-treated mice at 60 min.
Article Information
D.J.D. was supported in part by operating grants from the Juvenile Diabetes Research Foundation and the Canadian Diabetes Association and is a Senior Scientist of the Canadian Institutes for Health Research. Q.H. was supported by a research fellowship from the Canadian Diabetes Association.
We thank Human Genome Science scientists for the generous provision of Albugon and HSA used in these studies.




![FIG. 1. Albugon exhibits similar efficacy but lower potency than Ex-4 at the rat GLP-1R in vitro. A stable BHK cell line expressing the rat GLP-1R was pretreated for 5 min with 1 μmol/l exendin (9-39) [Ex(9-39)] or medium alone before a 10-min treatment with increasing concentrations of Ex-4, Albugon, or HSA. cAMP levels in lyophilized aliquots of cell extracts were measured by radioimmunoassay and used to calculate total cAMP content per well. Values are expressed as means ± SE and are representative of data from two independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 for Ex-4–vs. Albugon-treated cells.](https://ada.silverchair-cdn.com/ada/content_public/journal/diabetes/53/9/10.2337_diabetes.53.9.2492/3/m_zdb0090435300001.jpeg?Expires=1724210101&Signature=TVk2mMgXbecDxqfoIZwlizOBjcHjEYeURTZWTpC-zWCDjDRDQq1nIBiFbyZP0-t~gxJGeucXGq6BBRArQCUQllcn7pKbnawpb5-JSJFfGsA-tTdsWI7os6ZilRCPxF6WZxn-Hbi3fqwYmVMRq~F-FTxGX0ChdZ2T-8qHmCKTS9TVkHo-7DHH8GgZNwdjBY4F8USPe-I4rgPMoPPTF4t2z1SwT2mpxqjSfImxGqYoKzUuM4VQSYaZunB6MvlNF3tJ-d2-7u9UYk9qpelB86cB1Qlx-yYCqLfEkSfLMFNf2lZQsEbi2l9PEnd7x~Uw8Nb7UDFteSvY8qZf8cyKviMRnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)