Glucagon-like peptide (GLP)-1 inhibits food intake, acting both in the periphery and within the central nervous system. It is unclear if gut-derived GLP-1 can enter the brain, or whether GLP-1 from preproglucagon (PPG) cells in the lower brainstem is required to activate central GLP-1 receptors. Brainstem PPG neurons, however, have been poorly characterized, due to the difficulties in identifying these cells while viable. This study provides data on the electrical properties of brainstem PPG cells and their regulation by orexigenic and anorexigenic peptides.
Transgenic mice expressing Venus under control of the PPG promoter were used to identify PPG neurons in vitro in brainstem slice preparations for electrophysiological recordings.
The majority of PPG neurons were spontaneously active. Further electrical and molecular characterization revealed that GLP-1 receptor activation had no pre- or postsynaptic effect and that PPG neurons lack GLP-1 receptors. Similarly, they were unresponsive to PYY and ghrelin. In contrast, leptin rapidly and reversibly depolarized these neurons. Responses to electrical stimulation of the solitary tract suggest that PPG cells are mostly second-order neurons, receiving direct input from vagal afferent fibers. Both evoked and spontaneous excitatory postsynaptic currents were predominantly glutamatergic.
The study introduces PPG-promoter-Venus transgenic mice as a viable and important tool to study brainstem PPG cells. PPG neuron activity is directly modulated by leptin but was unaffected by other satiety or hunger peptides. Direct synaptic input from the solitary tract suggests that peripheral signals (including GLP-1) could modulate PPG cells via vagal afferents.
Glucagon-like peptide 1 (GLP-1) is an enteroendocrine hormone released postprandially from L-cells in the gut that stimulates insulin secretion and inhibits glucagon secretion. It also inhibits food and water intake and delays gastric emptying. Appetite-suppressive effects can be elicited by both peripheral and central application of GLP-1, raising questions about whether brain GLP-1 receptors respond primarily to gut-derived GLP-1 or to GLP-1 released within the central nervous system.
Whether GLP-1 levels even rise sufficiently after a meal to modulate central GLP-1 receptors remains controversial. Whereas Orskov et al. (1) argue that only GLP-1 receptors located in the area postrema (AP) and the subfornical area are accessible to peripheral GLP-1, Kastin et al. (2) claim that GLP-1 can readily diffuse across the blood-brain barrier. In any case, GLP-1 is rapidly inactivated in the circulation, with a half-life of <2 min. This might suggest that centrally active GLP-1 originates from the previously described subset of caudal nucleus tractus solitarius (NTS) neurons, which produce GLP-1 by posttranslational processing of preproglucagon (PPG) (3,–5).
Relatively little is known about these NTS PPG neurons. They project to various forebrain sites, including hypothalamic nuclei and the amygdala, as well as locally within the lower brainstem (3,–5). Within the hypothalamus, the densest innervation is observed in the paraventricular nucleus and the ventral part of the dorsomedial nucleus (3), regions that also exhibit strong expression of GLP-1 receptors (5).
The NTS receives input from sensory vagal neurons and is activated by gastrointestinal satiety signals such as gastric distension and luminal nutrients. c-Fos immunoreactivity has been observed in PPG neurons of the NTS after balloon gastric distension, suggesting that satiety stimuli can result in the activation of central GLP-1 pathways (6). In support of this hypothesis, peripheral administration of leptin induced c-Fos in NTS cells staining positive for PPG (7) and central application of leptin increased expression of preproglucagon in the NTS (8). It has also been reported that central administration of exendin (9–39), a GLP-1 receptor antagonist, strongly attenuated the reduction in food intake and body weight observed after intraventricular injection of leptin in rats (9). It therefore seems likely that PPG neurons are central to the integration of diverse satiety signals at the level of the NTS. This might include peptide YY (PYY3–36), which is co-released with GLP-1 from L-cells and has additive effects on food intake with GLP-1 (10), and ghrelin, which is released as a hunger peptide from the stomach and has been shown to attenuate the effects of GLP-1 on food intake (11).
At present, it is not clear whether there is a direct link between the action of peripheral GLP-1 and the activity of NTS PPG neurons. Instillation of nutrients into the duodenum, which would be expected to stimulate release of endogenous GLP-1, did not induce c-Fos in NTS PPG neurons (12). However, intravenous injection of exendin-4, a GLP-1 receptor agonist, induced c-Fos in the AP, a region containing a high density of GLP-1 receptors in catecholaminergic neurons (13). Based on these findings, Yamamoto et al. (13) speculated that PPG neurons in the NTS would receive input from these catecholaminergic AP cells and thus link peripheral GLP-1 to the release of GLP-1 from PPG cells, with downstream effects on central GLP-1 receptors within the hypothalamus and brainstem. It is also possible that PPG cells might send dendrites into the AP and outside the blood-brain barrier or that peripheral activation of GLP-1 receptors might modulate vagal afferent activity, which is relayed to the PPG cells via the solitary tract (14).
To date, the electrical properties and connectivity of the NTS PPG neurons remain largely uncharacterized because it has been virtually impossible to identify these neurons in brain slice preparations. Here we have used transgenic mice expressing the yellow fluorescent protein Venus under the control of the preproglucagon promoter (15) to identify this cell population and to characterize their electrical behavior, connectivity, and responsiveness to peptides implicated in the modulation of feeding behavior.
RESEARCH DESIGN AND METHODS
Transgenic animals.
Transgenic mice were used that expressed a modified yellow fluorescent protein (YFP-Venus) under the control of the preproglucagon promoter (15). Two founder strains, mGLU-V23-124 and mGLU-V50-144, created using mouse bacterial artificial chromosomes (BACs), were used interchangeably, since we observed no difference in the pattern of YFP expression in the brainstem (15). Animals were bred as heterozygotes on a C57/Bl6 background and were genotyped as described previously (15) before experimental use. All experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, with appropriate ethical approval.
Histology.
Mice were anesthetized with halothane and transcardially perfused with ice-cold heparinized saline followed by ice-cold 4% paraformaldehyde in phosphate buffer. The brain was removed and postfixed with 4% paraformaldehyde at 4°C overnight. Brains were cryoprotected with 30% sucrose in PBS, and 30-μm coronal brainstem sections were cut using a cryostat.
For immunofluorescence visualization of cholinergic cells, sections were incubated for 30 min in block solution (10% rabbit serum, 1% BSA, 0.1% triton X-100 in PBS), followed by overnight incubation with a polyclonal goat antibody against choline acetyltransferase (anti-ChAT, 1:1500; Chemicon) in block solution at 4°C. After five washes in PBS, the sections were incubated in the dark for 2 h at room temperature in a rabbit anti-goat IgG CY-3 conjugate (1:500; Sigma). Sections were mounted and cover-slipped with Vectashield. An equivalent protocol was used to visualize catecholaminergic cells with a polyclonal rabbit antibody against tyrosine-hydroxylase (anti-TH, 1:500; Santa Cruz). Block solution contained 10% sheep serum, and the secondary antibody was a sheep anti-rabbit IgG CY-3 conjugate (1:500; Sigma). Mounted sections were analyzed under a Nikon upright microscope with epifluorescence to visualize fluorescence of YFP and CY-3.
Single-cell RT-PCR.
Samples for RT-PCR and single-cell RT-PCR were harvested and amplified as described previously (16), using primers listed in Table 1. As negative controls for single-cell RT-PCR, reactions were routinely performed with H2O instead of DNA sample, with solution from pipettes that were inserted into the slice without recording from a cell and with samples that were not reverse-transcribed. For positive controls, PCRs were performed on a 1:100–1:1,000 dilution of brainstem cDNA. PCR products were verified by direct sequencing (MWG Biotech, London, U.K.).
PCR primers
| Primer . | PPG . | GLP-1 receptor . | Y2 receptor . |
|---|---|---|---|
| Outer forward primer | ATGAAGACCGTTTACATCGTGGC | TCAGAGACGGTGCAGAAATG | TGATCCGGAGCCGGAGCTCAT |
| Outer reverse primer | CTGGTGGCAAGGTTATCGAGA | GCAAACAGGTTCAGGTGGAT | AGGCGTGGAGGGGTAGCCAG |
| Inner forward | ACCAAGAGGAACCGGAAC | CAACCGGACCTTTGATGACT | CCAACCTGGCTGTGGCGGAT |
| Inner reverse primer | CCAAGTTCCTCAGCTATGGCG | CAAGGCGGAGAAAGAAAGTG | TCCCCAGGCCACTTCTCGGT |
| Outer product size (bp) | 492 | 458 | 750 |
| Inner product size (bp) | 186 | 292 | 361 |
| Primer . | PPG . | GLP-1 receptor . | Y2 receptor . |
|---|---|---|---|
| Outer forward primer | ATGAAGACCGTTTACATCGTGGC | TCAGAGACGGTGCAGAAATG | TGATCCGGAGCCGGAGCTCAT |
| Outer reverse primer | CTGGTGGCAAGGTTATCGAGA | GCAAACAGGTTCAGGTGGAT | AGGCGTGGAGGGGTAGCCAG |
| Inner forward | ACCAAGAGGAACCGGAAC | CAACCGGACCTTTGATGACT | CCAACCTGGCTGTGGCGGAT |
| Inner reverse primer | CCAAGTTCCTCAGCTATGGCG | CAAGGCGGAGAAAGAAAGTG | TCCCCAGGCCACTTCTCGGT |
| Outer product size (bp) | 492 | 458 | 750 |
| Inner product size (bp) | 186 | 292 | 361 |
Electrophysiology.
Coronal (200 μm) or horizontal (250 μm) brainstem slices were obtained from adult (>8 weeks) transgenic mice of either sex after halothane anesthesia and dissection in ice-cold low Na+ solution containing (in mmol/l): 200 sucrose, 2.5 KCl, 28 NaHCO3, 1.25 NaH2PO4, 3 pyruvate, 7 MgCl2, 0.5 CaCl2, and 7 glucose (pH 7.4). After recovery at 34°C for 30 min in a solution containing (in mmol/l) 118 NaCl, 3 KCl, 25 NaHCO3, 1.2 NaH2PO4, 7 MgCl2, 0.5 CaCl2, and 2.5 glucose (pH 7.4), slices were kept at 34°C in artificial cerebrospinal fluid (ACSF) of the following composition (in mmol/l): 118 NaCl, 3 KCl, 25 NaHCO3, 1 MgCl2, 2 CaCl2, and 10 glucose (pH 7.4). Patch pipettes were pulled from thin-walled borosilicate capillaries (3–6 MΩ; Clark Electromedical Instruments, Pangbourne, U.K.) with a horizontal puller (Zeitz, Germany). Electrodes were filled with (in mmol/l) 120 K-gluconate, 5 HEPES, 5 BAPTA, 1 NaCl, 1 MgCl2, 1 CaCl2, and 2 K2ATP, pH 7.2. For perforated-patch whole-cell recording, solubilized amphotericin B (Sigma) was added to the pipette solution (final concentration ∼137.5 μg/ml).
Recordings were carried out in ACSF at 28–32°C. Experimental solutions were constantly bubbled with 95% O2/5% CO2. Most drugs were directly added to the ACSF. 6,7-Dinitroquinoxaline-2,3-dione (DNQX) was prepared as a 20 mmol/l stock solution in DMSO. Tetrodotoxin was prepared as a 1 mmol/l stock solution in Na-citrate buffer. The recording chamber (volume 2 ml) was perfused with ACSF at a rate of 4–5 ml/min. Drugs were applied either within the ACSF perfusing the bath (4–5 ml/min) or locally via pressure ejection from a glass pipette (opening diameter 5–10 μm) facing the recorded cell positioned at a distance of ∼100 μm. Exendin-4 and PYY3–36 were obtained from Tocris Bioscience (Bristol, U.K.). Ghrelin and GLP-17–37 were obtained from Sigma (Gillingham, U.K.). Leptin was a kind gift from Novo Nordisk AS (Copenhagen, Denmark).
Recordings were performed in both voltage-clamp and current-clamp mode using an EPC-9 amplifier and Pulse/Pulsefit software (Heka Elektronik, Lambrecht, Germany). Currents or membrane potentials were filtered at 1 kHz and digitized at 4 kHz. Membrane resistance was monitored with 200 ms current or voltage pulses every 20 s. Current-voltage relationships were obtained either by analyzing hyperpolarizing and depolarizing 200-ms voltage or current steps, or by holding the cell at −20 mV for a minimum of 30 s and then applying a voltage ramp from −20 to −120 mV over 700 ms.
For analysis of firing frequency, membrane potential, input resistance, and spontaneous excitatory postsynaptic current frequency, the mean value over 3 min before addition of the drug and the mean over the last 3 min before drug removal were compared. For leptin and glutamate, which were washed off quickly to ascertain reversibility, peak responses were compared to the mean before drug application.
Solitary tract stimulation.
A concentric bipolar electrode (150 μm diameter) was placed in the solitary tract of horizontal brainstem slices ∼2–4 mm away from the fluorescent cell population. Either single shocks (every 10 s) or bursts of five shocks (50 Hz; every 20 s) were delivered from a stimulus isolator (DS2A; Digitimer) driven by the EPC-9 amplifier. Each stimulus had a duration of 20 μs and an amplitude of 40–70 V. Amplitude was increased using single stimuli during individual recordings until a further increase in stimulus amplitude had no further effect on excitatory post synaptic current (EPSC) amplitude. Latency was determined as the delay between the onset of the stimulus artifact and the onset of the EPSC, as described by Doyle and Andresen (17). Synaptic jitter was determined as the standard deviation of the latency for >20 successive single shocks at 0.1 Hz.
Statistical analysis.
Data are given as mean ± 1 SEM. Statistical significance was tested using one-way ANOVA followed by the post hoc Tukey's test, unless stated otherwise. P values of <0.05 and <0.01 were taken to indicate that the data were significantly different.
RESULTS
Electrical properties of NTS preproglucagon neurons.
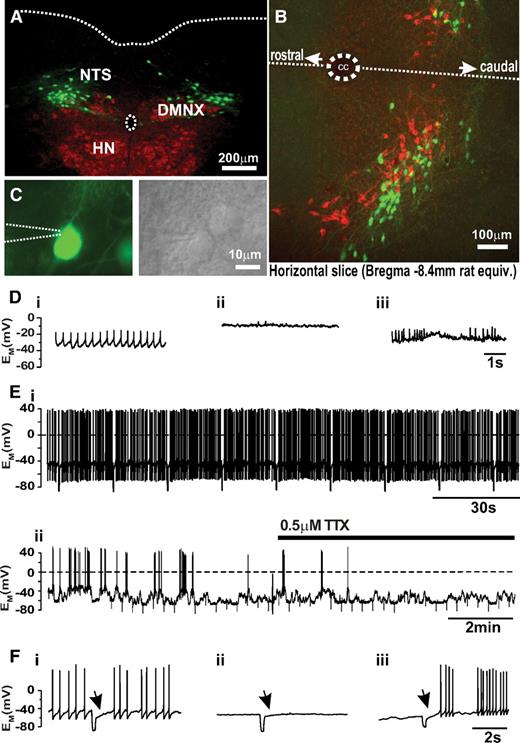
PPG neurons in acute brainstem slices were readily identifiable by their Venus fluorescence. The majority of Venus-positive cells in these slices were found within the caudal NTS, although additional cell bodies were located further ventrally, e.g., in the reticular area. Venus-positive cells had no clear distinguishing morphological features, and their distribution overlapped with various NTS neuron populations. They were distinct both from ChAT-positive cells in the dorsal vagal motor nucleus (DMNX; Fig. 1,A) and from tyrosine hydroxylase (TH)-positive cells in the NTS—a population that includes adrenergic, noradrenergic, and dopaminergic neurons (Fig. 1 B).
Basic properties of PPG neurons of the NTS. A: Photomicrograph showing the location of PPG-Venus neurons (green fluorescence) in relation to the cholinergic (red fluorescence) dorsal vagal nucleus (DMNX) and hypoglossal nucleus (HN) in a 30-μm coronal brainstem slice at position Bregma −7.75 mm. The central canal and the dorsal border of the brainstem are indicated by dotted lines. B: Horizontal brainstem slice demonstrating the catecholaminergic cells (red fluorescence signifying anti-TH immunoreactivity) and PPG cells (green fluorescence) are two completely distinct cell populations that occupy overlapping areas of the NTS. The dotted line indicates the midline. CC, central canal. Confocal analysis of selected regions from these slices suggests that there is no direct contact between TH-positive and PPG cells (data not shown). C: Individual PPG neurons were identified by Venus fluorescence (top, patch pipette position indicated by dotted lines) and recordings were established under DIC illumination (bottom). D: In the cell-attached configuration while waiting for perforation, the firing phenotype of the neuron was observed: i, regular firing; ii, silent; iii, burst firing. E: Typical perforated-patch current-clamp recordings from regular firing neuron (i) and burst firing cell (ii). The fluctuations in membrane potential underlying the burst firing behavior persisted in TTX. F: injection of a −40 pA current pulse for 200 ms elicited an A-type K+ current (arrow) upon termination of the pulse in all three cell types. (A high-quality digital representation of this figure is available in the online issue.)
Basic properties of PPG neurons of the NTS. A: Photomicrograph showing the location of PPG-Venus neurons (green fluorescence) in relation to the cholinergic (red fluorescence) dorsal vagal nucleus (DMNX) and hypoglossal nucleus (HN) in a 30-μm coronal brainstem slice at position Bregma −7.75 mm. The central canal and the dorsal border of the brainstem are indicated by dotted lines. B: Horizontal brainstem slice demonstrating the catecholaminergic cells (red fluorescence signifying anti-TH immunoreactivity) and PPG cells (green fluorescence) are two completely distinct cell populations that occupy overlapping areas of the NTS. The dotted line indicates the midline. CC, central canal. Confocal analysis of selected regions from these slices suggests that there is no direct contact between TH-positive and PPG cells (data not shown). C: Individual PPG neurons were identified by Venus fluorescence (top, patch pipette position indicated by dotted lines) and recordings were established under DIC illumination (bottom). D: In the cell-attached configuration while waiting for perforation, the firing phenotype of the neuron was observed: i, regular firing; ii, silent; iii, burst firing. E: Typical perforated-patch current-clamp recordings from regular firing neuron (i) and burst firing cell (ii). The fluctuations in membrane potential underlying the burst firing behavior persisted in TTX. F: injection of a −40 pA current pulse for 200 ms elicited an A-type K+ current (arrow) upon termination of the pulse in all three cell types. (A high-quality digital representation of this figure is available in the online issue.)
Electrophysiological recordings were established from fluorescent cells in the NTS under differential interference contrast (DIC) optics (Fig. 1 C), and cytoplasm was harvested at the end of the recordings and subjected to single-cell RT-PCR for preproglucagon. Eight out of eight fluorescent cells were positive for preproglucagon and nine out of nine control cells were negative, verifying both the correct targeting of the transgene and that preproglucagon is still transcribed in PPG cells expressing Venus.
In perforated patch recordings, it was evident soon after sealing the electrode onto an individual cell that the intrinsic electrical activity of PPG neurons could be divided into three types: regular firing, silent, and burst firing (Fig. 1,D). These phenotypes persisted under stable perforated-patch conditions. Of 135 recorded neurons, the majority (n = 99) were spontaneously active with a mean firing frequency of 1.9 ± 0.2 Hz. These neurons exhibited a resting membrane potential of −51 ± 1 mV (Fig. 1,D and E), with an average input resistance of 1.05 ± 0.03 GΩ and capacitance of 28 ± 1 pF. Another 11 cells were electrically silent, with a resting potential of −55 ± 2 mV, an input resistance of 0.84 ± 0.09 GΩ, and a capacitance of 27 ± 3 pF (Fig. 1,D and F). Action potentials could be elicited in these cells by current injections of 14 ± 1 pA (n = 9). The remaining 25 neurons showed burst-firing activity, with spontaneous fluctuations of the membrane potential by almost 20 mV (Fig. 1,D and E) that persisted in 0.5 μmol/l tetrodotoxin (n = 5). These cells had a whole-cell capacitance of 21 ± 1 pF. In all three cell types, injection of hyperpolarizing current pulses (200 ms) caused a delay of ∼1 s before the return of spontaneous activity (Fig. 1 F), indicating that PPG neurons express A-type K+ currents. All further experiments for this study were performed on the spontaneously active cell population.
Synaptic inputs on PPG neurons.
The location of PPG neurons in the NTS, a region with an intact blood-brain barrier, suggests that these cells would be predominantly modulated by synaptic inputs. To investigate excitatory synaptic inputs onto PPG neurons, cells were held at −70 mV in standard whole-cell recordings, under which conditions EPSCs were observed as inward currents. Spontaneous EPSCs (sEPSCs) occurred at a frequency of 3.0 ± 0.5 Hz with an amplitude of 23 ± 2 pA (n = 21) and were largely glutamatergic, as demonstrated by their inhibition by kynurenic acid (1 mmol/l) or DNQX (10 μmol/l; Fig. 2,A and B). In support of the idea that PPG neurons receive direct glutamatergic input, glutamate (0.1 mmol/l) triggered membrane depolarization and a reduction in the membrane resistance. Both actions were reversed by DNQX (20 μmol/l), indicating the involvement of non-NMDA type glutamate receptors (Fig. 2 C and D).
PPG neurons have postsynaptic ionotropic glutamate receptors. A: Voltage-clamp recording at a holding potential of −70 mV demonstrating spontaneous excitatory postsynaptic current (EPSC) activity and its inhibition by the glutamate receptor antagonist kynurenic acid (Kyn; 1 mmol/l). B: Mean data from recordings as shown in A and from experiments with the non-NMDA receptor antagonist DNQX (10 μmol/l). Mean EPSC frequency was determined over 3 min under each condition. **P < 0.01 against control. C: Bath application of 0.1 mmol/l glutamate during a perforated patch clamp recording caused a reversible depolarization by ∼40 mV in this PPG neuron. D: Mean effects of glutamate and glutamate plus DNQX (20 μmol/l) on the input resistance (RM) and membrane potential (EM) of PPG neurons. Note that DNQX almost completely abolishes the glutamate effect. **P < 0.01 against control; ##P < 0.01 against glutamate. Numbers of cells tested (n) are given above the bars.
PPG neurons have postsynaptic ionotropic glutamate receptors. A: Voltage-clamp recording at a holding potential of −70 mV demonstrating spontaneous excitatory postsynaptic current (EPSC) activity and its inhibition by the glutamate receptor antagonist kynurenic acid (Kyn; 1 mmol/l). B: Mean data from recordings as shown in A and from experiments with the non-NMDA receptor antagonist DNQX (10 μmol/l). Mean EPSC frequency was determined over 3 min under each condition. **P < 0.01 against control. C: Bath application of 0.1 mmol/l glutamate during a perforated patch clamp recording caused a reversible depolarization by ∼40 mV in this PPG neuron. D: Mean effects of glutamate and glutamate plus DNQX (20 μmol/l) on the input resistance (RM) and membrane potential (EM) of PPG neurons. Note that DNQX almost completely abolishes the glutamate effect. **P < 0.01 against control; ##P < 0.01 against glutamate. Numbers of cells tested (n) are given above the bars.
One potential source of excitatory glutamatergic inputs are vagal sensory neurons. Previous studies in horizontal brainstem slices have shown that catecholaminergic NTS neurons receive direct monosynaptic input from afferent vagal fibers in the solitary tract (18,–20). We therefore used this recording configuration to examine whether PPG neurons are also responsive to solitary tract stimulation. Tract stimulation reliably evoked EPSCs in PPG neurons with a mean latency of 3.2 ± 0.3 ms and a mean peak amplitude of 126 ± 17 pA (n = 14) (Fig. 3 A and B).
Stimulation of the solitary tract evokes glutamate EPSCs in PPG neurons. A: Voltage clamp recording from a PPG neuron in a horizontal slice at a holding potential of −70 mV. Repetitive stimulation of the solitary tract (five pulses at 50 Hz every 20 s) evoked EPSCs of progressively smaller amplitude, small failure rate, and low jitter (i.e., mono-synaptic) in most of these cells. A total of 10 consecutive traces are overlaid. B: Some cells showed higher failure rates and larger jitter. A total of 10 consecutive traces are overlaid. C: All cells tested showed a marked reduction in EPSC amplitude in the presence of glutamate receptor antagonists kynurenic acid (kyn) or DNQX (gray trace in i and ii). D: Mean data from recordings as shown in C. **P < 0.01 against control. Numbers of cells tested (n) are given above the bars.
Stimulation of the solitary tract evokes glutamate EPSCs in PPG neurons. A: Voltage clamp recording from a PPG neuron in a horizontal slice at a holding potential of −70 mV. Repetitive stimulation of the solitary tract (five pulses at 50 Hz every 20 s) evoked EPSCs of progressively smaller amplitude, small failure rate, and low jitter (i.e., mono-synaptic) in most of these cells. A total of 10 consecutive traces are overlaid. B: Some cells showed higher failure rates and larger jitter. A total of 10 consecutive traces are overlaid. C: All cells tested showed a marked reduction in EPSC amplitude in the presence of glutamate receptor antagonists kynurenic acid (kyn) or DNQX (gray trace in i and ii). D: Mean data from recordings as shown in C. **P < 0.01 against control. Numbers of cells tested (n) are given above the bars.
Further assessment of whether the observed responses were monosynaptic or polysynaptic was performed by examining their failure rate, latency, and jitter (the variability of the latency). Of the cells, 11 of 14 had a failure rate of <5%, and the majority of cells displayed a jitter of <300 μs, suggesting that the responses are predominantly, but not entirely, explained by monosynaptic connections. DNQX (10 μmol/l) completely blocked EPSCs evoked by tract stimulation in all cells tested (n = 7), and kynurenic acid (1 mmol/l) reduced their amplitude by 58% (n = 3; Fig. 3,C and D). In 10 of 14 cells, the EPSC amplitude became progressively smaller in response to a burst of five shocks at 50 Hz (Fig. 3 A, Ci, and Cii). These results suggest that the majority of PPG cells receive direct synaptic input from the solitary tract (and are thus second-order neurons) and that both the direct and indirect excitatory inputs are glutamatergic.
Effects of gut hormones on PPG neurons.
GLP-17–37 (100–200 nmol/l) had no effect on the resting membrane potential, membrane resistance, or firing frequency of PPG cells (n = 6; Fig. 4,A and B) and did not affect the current response to voltage ramps between −20 and −120 mV. Similarly, the stable GLP-1 analog exendin-4 (100 nmol/l) failed to alter the membrane potential, membrane resistance, or firing frequency of these cells (n = 5). These findings suggested that NTS PPG neurons do not express functional GLP-1 receptors. This conclusion was further supported by single-cell RT-PCR analysis of 12 cells from which cytoplasm was harvested at the end of the recordings. PPG expression was detected in all 12 cells, thus verifying their phenotype, but no cell tested positive for GLP-1 receptor mRNA. By contrast, GLP-1 receptor mRNA was detected successfully in a 1:1,000 dilution of mouse brainstem cDNA (Fig. 4 C).
PPG neurons are not sensitive to gut peptides GLP-1 and PYY. A: Perforated patch clamp recording in current clamp demonstrating the lack of effect of 100 nmol/l GLP-1 on PPG cell activity. The top trace shows the instantaneous firing frequency over the course of the recording, and the bottom traces show brief periods of the original recording at time points indicated by arrows. B: Mean firing frequency in the absence and presence of 100 nmol/l GLP-1 or 100 nmol/l of the GLP-1 receptor agonist exendin-4. C: Typical single-cell RT-PCR analysis for PPG and the GLP-1 receptor (GLP-1R) for three PPG neurons and controls. The 2% agarose gel demonstrating that the 186-bp PCR product for PPG and the 292-bp PCR product for GLP-1R can be obtained from brainstem cDNA (BS 1:1,000 dilution; positive control) with the primers specified in Table 1. In contrast, cytoplasm extracted from single cells showing Venus fluorescence (1,–3) was only positive for PPG, but not GLP-1R. PIP, pipette solution without cytoplasm extracted from cell (negative control). D: Neither exendin-4 nor the gut peptide PYY had any effect on spontaneous EPSCs in PPG neurons. Left: Typical voltage-clamp recordings in the absence or presence of exendin-4. Right: Typical recordings in the absence or presence of PYY. E: Mean data from recordings as shown in B for frequency and amplitude of spontaneous EPSCs. F: The peptide ghrelin (100 nmol/l), released from stomach as orexinergic signal, had no effect on membrane potential (EM), input resistance (RM), or action potential frequency. Numbers of cells tested (n) are given above the bars.
PPG neurons are not sensitive to gut peptides GLP-1 and PYY. A: Perforated patch clamp recording in current clamp demonstrating the lack of effect of 100 nmol/l GLP-1 on PPG cell activity. The top trace shows the instantaneous firing frequency over the course of the recording, and the bottom traces show brief periods of the original recording at time points indicated by arrows. B: Mean firing frequency in the absence and presence of 100 nmol/l GLP-1 or 100 nmol/l of the GLP-1 receptor agonist exendin-4. C: Typical single-cell RT-PCR analysis for PPG and the GLP-1 receptor (GLP-1R) for three PPG neurons and controls. The 2% agarose gel demonstrating that the 186-bp PCR product for PPG and the 292-bp PCR product for GLP-1R can be obtained from brainstem cDNA (BS 1:1,000 dilution; positive control) with the primers specified in Table 1. In contrast, cytoplasm extracted from single cells showing Venus fluorescence (1,–3) was only positive for PPG, but not GLP-1R. PIP, pipette solution without cytoplasm extracted from cell (negative control). D: Neither exendin-4 nor the gut peptide PYY had any effect on spontaneous EPSCs in PPG neurons. Left: Typical voltage-clamp recordings in the absence or presence of exendin-4. Right: Typical recordings in the absence or presence of PYY. E: Mean data from recordings as shown in B for frequency and amplitude of spontaneous EPSCs. F: The peptide ghrelin (100 nmol/l), released from stomach as orexinergic signal, had no effect on membrane potential (EM), input resistance (RM), or action potential frequency. Numbers of cells tested (n) are given above the bars.
Although these findings exclude a direct effect of GLP-1 on PPG cells, we also examined whether presynaptic GLP-1 receptors might modulate synaptic inputs onto PPG neurons. However, local application of 10 nmol/l exendin-4 had no significant effect on either the amplitude or frequency of sEPSCs (n = 5; Fig. 4 D and E).
In a further series of experiments, we examined the effects of two more gut-derived peptides involved in appetite control. PYY3–36 (100 nmol/l), which is co-released with GLP-1 from enteroendocrine L-cells, was without effect on membrane properties and sEPSC frequency or amplitude in voltage-clamp recordings (n = 6; Fig. 4,D and E). In accordance with these results, no Y2 receptor message was detected with single-cell RT-PCR analysis of six PPG neurons. Ghrelin (100 nmol/l), which is released from the stomach as an orexinergic signal, also failed to elicit any significant change in membrane potential, membrane resistance, or spontaneous action potential firing rate in PPG cells (n = 5; Fig. 4 F).
Effects of leptin on PPG neurons.
To investigate whether the anorexigenic peptide leptin has direct effects on PPG neuron activity, we tested its effect in perforated patch current clamp recordings. Leptin (20 nmol/l) applied locally caused a depolarization of 9 ± 2 mV that was accompanied by an increase in firing frequency and a decrease in membrane resistance in eight of eight PPG cells (Fig. 5). This response was reversible upon washout of leptin.
Leptin depolarizes PPG neurons. A: Response to local application of 20 nmol/l leptin. Top trace shows the action potential firing rate (FR), and the bottom trace shows the full current-clamp recording. B: Periods of the recording indicated in A by a, b, and c at an extended timescale. Leptin caused a depolarization of the membrane potential (EM), decrease in membrane resistance (RM), and increase in action potential firing rate (FR). C: Mean data from eight cells showing the peak response for each parameter. *P < 0.05 against control; **P < 0.01 against control. Numbers of cells tested (n) are given above the bars.
Leptin depolarizes PPG neurons. A: Response to local application of 20 nmol/l leptin. Top trace shows the action potential firing rate (FR), and the bottom trace shows the full current-clamp recording. B: Periods of the recording indicated in A by a, b, and c at an extended timescale. Leptin caused a depolarization of the membrane potential (EM), decrease in membrane resistance (RM), and increase in action potential firing rate (FR). C: Mean data from eight cells showing the peak response for each parameter. *P < 0.05 against control; **P < 0.01 against control. Numbers of cells tested (n) are given above the bars.
DISCUSSION
Activation of PPG neurons in the NTS has been linked to a reduction of food intake in a number of studies that demonstrated c-Fos expression in these cells in response to satiety signals such as gastric distension, consumption of sweetened milk, or elevated leptin levels (6,–8,21,22). However, because of the inability to identify PPG neurons in live tissue, nothing has been known about their electrical properties, or the direct modulation of their activity. Our results describe for the first time the electrical properties of PPG neurons located in the caudal NTS and their direct activation by leptin.
General properties.
Under resting conditions, the majority of PPG neurons in the NTS were spontaneously active, firing action potentials at a frequency of ∼2 Hz. In this respect, they resemble the neighboring population of paraventricular nucleus–projecting catecholaminergic neurons that were also reported to be spontaneously active (18). POMC neurons in the NTS, by contrast, receive visceral afferent input but are electrically silent unless stimulated (19). At present, we do not know how the level of spontaneous activity of PPG neurons translates to their release of GLP-1 from nerve terminals. Recent studies by Wan et al. (23,24) investigating the effects of GLP-1 and GLP-1 antagonists on dorsal vagal neurons using an equivalent preparation in rat found no evidence of tonic GLP-1 release onto pancreas-projecting vagal motoneurons. This might be taken as an indication that the resting activity of the PPG neurons is not sufficient to induce local release of GLP-1, although whether there is any tonic release of GLP-1 at the level of the hypothalamus remains unknown.
NTS PPG neurons display A-type K+ currents, a feature that they share with other second-order NTS neurons such as TH-positive cells (18) as well as vagal preganglionic neurons of the adjacent dorsal vagal motor nucleus (DMNX [25]). In accordance with the hypothalamic projection of PPG neurons, A-type K+ currents have been described previously in NTS neurons projecting to the paraventricular nucleus, but not those projecting to the caudal ventrolateral medulla (26).
Most PPG neurons in the NTS were found to be second-order neurons that receive strong glutamatergic synaptic input from the solitary tract and express non-NMDA type glutamate receptors (27). These excitatory inputs would be predominantly from visceral vagal afferents, in line with the finding that c-Fos is induced in GLP-1 neurons upon gastric distension (6).
Lack of GLP-1 modulation of activity.
Rises in peripheral GLP-1 have been linked to activation of neurons located in the NTS. Our electrophysiological and expression data provide direct evidence that NTS PPG neurons do not have functional GLP-1 receptors. While we cannot exclude the possibility that they might respond to activation of presynaptic GLP-1 receptors, this seems unlikely to be the case because we observed no effect of GLP-1 or exendin-4 on firing rate or spontaneous synaptic activity. Thus, even if peripheral GLP-1 was able to cross the blood-brain barrier, it seems unlikely that it would have a local effect on the activity of PPG neurons. It remains possible, however, that potential connections between GLP-1–sensitive cells in the area postrema and PPG cells in the NTS might have been lost in the slice preparation.
An alternative pathway by which peripheral GLP-1 might modulate PPG cell activity in the NTS is via GLP-1 receptors on vagal afferent neurons (14). GLP-1 receptor mRNA has been detected in the inferior ganglion of the afferent vagus (the nodose ganglion), and electrical activity in the vagus nerve was shown to respond to GLP-1 application (14). It is therefore possible that NTS PPG cells, which appear to be predominantly second-order neurons with regard to solitary tract stimulation, would relay this vagal signal, resulting in central GLP-1 release in the brainstem and hypothalamus. In support of this hypothesis, it was shown that appetite suppression and arcuate nucleus expression of c-Fos elicited by peripheral GLP-1 were attenuated by vagotomy (28). The idea that circulating GLP-1 is detected by peripheral rather than central GLP-1 receptors was also suggested by the finding that intraperitoneal but not intracerebroventricular application of exendin (9–39) antagonized the appetite suppressive effects of peripheral GLP-1 (29). However, direct evidence that NTS PPG neurons are activated via a vagal pathway when GLP-1 is released postprandially from gastrointestinal L-cells is still lacking.
GLP-1 receptors have been reported on area postrema neurons as well as in many other locations throughout the brainstem (5,30). Electrophysiological studies in brainstem slices have demonstrated that pancreas-projecting dorsal vagal neurons are both directly and indirectly modulated by GLP-1 (23,24). The lack of GLP-1 responsiveness of PPG cells in the NTS suggests that PPG neurons do not receive inputs from the same cells as pancreas-projecting dorsal vagal neurons. One possibility is that they are actually the source of the GLP-1 that affects vagal output.
Effects of other peripheral satiety and hunger peptides on PPG cell activity.
PYY3–36 is a satiety peptide (31), released from intestinal L-cells in parallel with GLP-1. Its peripheral administration has been shown to increase c-Fos expression in cell bodies in the area postrema and NTS, including populations that were negative for tyrosine hydroxylase and might therefore overlap with PPG cells (32). In brainstem slices, however, we were unable to elicit either pre- or postsynaptic responses to PYY3–36 in NTS PPG neurons. Peripheral injections of PYY3–36 and GLP-1 receptor agonists have synergistic effects on appetite suppression in rats and mice (10). Destruction of vagal afferent neurons using capsaicin prevented the reduction of food intake by exendin-4, but not that by PYY3–36 (33), suggesting that unlike GLP-1, the effect of PYY does not require intact sensory neurons. Although the PYY3–36-responsive receptor type, Y2, has been identified in a number of brainstem regions including the area postrema and NTS (34), it remains unclear whether the appetite suppressive action of PYY3–36 depends on NTS PPG neurons.
The adipocyte-derived hormone leptin directly and rapidly increased the electrical activity of PPG neurons. The electrophysiological data suggest that this is mediated by the activation of a depolarizing conductance, in contrast to the inhibitory effect of leptin on K+ channels described previously in ventromedial hypothalamic neurons (35). These results provide a potential explanation for previous findings that c-Fos immunoreactivity in PPG neurons is induced by leptin exposure (7). They also support previous observations that PPG neurons in mice express leptin receptor mRNA (9) and that leptin activates STAT3 phosphorylation, a marker of direct leptin action, in PPG neurons in mice, but not rats (21). The immediate effect of leptin on PPG cell electrical activity suggests that a component of the effect of this hormone, normally considered as a longer-term regulator of food intake, is mediated by enhancing postprandial satiety signals involving NTS PPG neurons. By contrast, the orexigenic peptide ghrelin (36), which is released from the stomach in anticipation of food ingestion, failed to affect the electrical activity of PPG neurons. Thus, although ghrelin has been reported to attenuate GLP-1–induced inhibition of food intake and gastric emptying (11), our results suggest that this does not happen at the level of the NTS PPG neurons.
In conclusion, our data provide a first functional in vitro characterization of PPG neurons in the caudal NTS—a study made possible by the development of Venus-tagged GLP-1 mice (15), which reliably express a fluorescent marker in PPG cells in the brainstem. Interestingly, we find that the activity of NTS PPG neurons is rapidly and directly modulated by leptin but is unaffected by GLP-1, PYY, or ghrelin. These findings have important implications for our understanding of the role of NTS PPG neurons in relaying appetite signals between the periphery and the hypothalamus.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
ACKNOWLEDGMENTS
This study was supported by the Medical Research Council, U.K. (K.H. and S.T. Ref. G0600928), and the Wellcome Trust (FMG Ref. 088357 and FR Ref. 084210).
No potential conflicts of interest relevant to this article were reported.
K.H. researched most data, contributed to discussion, and edited the manuscript; F.R. contributed to study design and edited the manuscript; F.M.G. contributed to the study design and edited the manuscript; and S.T. designed the study, researched some data, and wrote the manuscript.