December 16, 2019 feature
Plasma ionization-based 3-D titania nanofiber-like webs to enhance bioreactivity and osteoconductivity of biomaterials

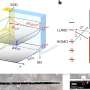
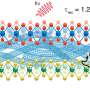
In a new study published on Scientific Reports, Mohammad-Hossein Beigi and a research team in the departments of Engineering and Applied Science and Cellular Biotechnology in Canada and Iran described a new method to form biocompatible biomaterials for bone tissue engineering. They engineered web-like, three-dimensional (3-D) Titania nanofibrous coatings using high intensity laser-induced reverse transfer (HILIRT). The team first demonstrated the mechanism of ablation and Titanium (Ti) deposition on glass substrates using multiple picosecond laser pulses in ambient air to compare theoretical predictions with experimental results. They examined the performance of glass samples developed by coating titania nanofibrous structures through varied laser pulse durations, using methods such as scanning electron microscopy (SEM).
To understand interactions between the new material surface and biological cells, Beigi et al. explored interactions of human bone-derived mesenchymal stem cells (BMSCs) cultured on the new biomaterials. For this, they used a variety of in-lab tests including a colorimetric method to understand cell metabolic activity (MTS assay), immunocytochemistry, protein adsorption and absorption analyses. The results showed significantly improved biocompatibility in laser-treated samples compared to untreated substrates. Beigi et al. modified their HILIRT technique by decreasing the pulse duration and generating titania nanofibers with denser structures during advanced materials engineering. According to their findings, the density of nanostructures and the concentration of coated nanofibers played a critical role to generate bioactivity in the treated samples by inducing early differentiation of BMSCs (bone-derived mesenchymal stem cells) to form bone tissue via osteogenic differentiation (bone formation).
Bioengineers are rapidly developing new techniques of bone tissue engineering (BTE) for bone regeneration; to improve the existing "gold standards" of bone autograft and allograft methods in regenerative medicine. Disadvantages of the existing techniques include donor site morbidity and limited nutrient supplements during bone regeneration. Bone tissue engineering (BTE) is a promising research direction to facilitate bone growth and repair, even in large-scale skeletal defects. Researchers aim to use stem cells with BTE due to their self-renewing capabilities alongside stem cell differentiation, to form a variety of tissue types. Since the physical and chemical properties of a material surface can influence the viability of human mesenchymal stem cells (hMSCs) for self-restoration, differentiation and proliferation. Materials and cells can therefore work together in applications of BTE to provide a desired platform for osseointegration during bone remodeling.

Research teams had previously used several techniques to produce BTE material surfaces including sol-gel, hydrothermal 106, electrospinning and 3-D printing; however, selecting an ideal method remains a challenge. For instance, artificial biomaterials must interact effortlessly with physiological fluids and assimilate with hard and soft surrounding tissues to maintain cellular activity for superior biocompatibility. Materials scientists and bioengineers had used titanium and its alloys for orthopedic implants, allowing titanium nanoparticle (NP)-based osteogenesis of dental pulp stem cells and adipose-derived stem cells. Laser surface modification methods can modify material surfaces for improved surface biocompatibility; where the HILIRT method had previously shown potential to engineer lab-on-a-chip components and other biocompatible biomaterials. Scientists can alter laser parameters to manipulate material surfaces to assist cell differentiation.
In the present work, Beigi et al. investigated effects of laser pulse duration on material surfaces using the HILIRT method and tested the biological behavior of synthetic biomaterials using materials characterization and biological tests in the lab. They investigated cell-material contact on material surfaces using gene expression, mineralization and protein interaction studies. The scientists developed a nanofibrous titania (NFTi) thin film and soaked it in simulated body fluid (SBF) to form hydroxyapatite (HA)-like layer structures and identified the material surface modifications using water contact angle (CA), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) analysis, micro-Raman and X-ray diffraction (XRD) spectroscopies.

They deposited NFTi (nanofibrous titania) structures at different pulse durations to form laser nanofiber coated smooth surfaces and tested the chemical and physical composition of the resulting advanced materials. When they decreased pulse duration, the titanium weight percentage increased, and the scientists observed the temperature of the irradiated zone to be significantly higher for a shorter pulse duration of 150 picoseconds (ps) compared with 5 nanoseconds (ns) and 30 ns. The decreased pulse duration transmitted power to the target in a shorter time, causing the heat affected zone (HAZ) to have a higher temperature, allowing a denser plasma plume to form more NFTi structures on a glass substrate. Decreasing the laser pulse duration created more biocompatible Ti nanofibers with a higher content of HA(hydroxyapatite)-like substance sedimentation on the samples.
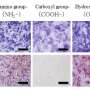
Using phase-contrast microscopy images of fibroblast-like BMSCs on titania-coated glass surfaces, Beigi et al. observed normal cell morphology. They measured water contact angles of droplets of water on the material specimens and conducted cytotoxicity tests with MTS assays on stem cells grown on NFTi coatings. The materials coated with NFTi for 150 ps showed the highest absorbance rate (known as the S1 group) with subsequently high rates of cell viability, cell adhesion and metabolic activity. When the researchers used immunofluorescent staining to observe cell migration, the S1 sample (with NFTi coating for 150 ps) showed higher rates of cell migration. To confirm stem cell (BMSC) differentiation, the scientists investigated osteogenic-related gene expression with RUNX2, collagen I, osteopontin and osteonectin genes, using quantitative qRT-PCR. Among the samples, S1 samples indicated significantly higher relative expression for all osteogenic-related genes.

To confirm mineralization, the scientists used alizarin red followed by soluble Ca nodules color absorbance quantification, to observe high levels of mineralization on all samples on days seven and 14. The team investigated surface protein absorption potential, protein-ion biocomplex formation and biocomplex cell uptake to demonstrate highest levels of protein-ion biocomplex formation on the S1 samples.
In this way, Mohammad-Hossein Beigi and colleagues used the HILIRT method to achieve high surface bioreactivity, osteogenesis and osseointregration of NFTi-BMSCs. The surface character of the new materials allowed protein and biomolecule interactions to stimulate cell adhesion, mineralization and osteogenesis for faster and more suited osseointegration in vivo and in vitro. The scientists engineered nanofiber mesh-like scaffolds using titanate to allow vascularization, protein attachment, cell proliferation and cell attachment on the substrate. Such microporous surfaces can promote nutrition diffusion, vascularization and blood flow due to improved biomechanical strength. In addition, the hydrophilic surface property; verified using water contact angle measurements, facilitated cell-ECM adhesion to improve cell binding and vigorous tissue growth.

The S1 sample (NFTi, 150 ps) developed in this work generated the best surface bioreactivity for bone regeneration or bone replacement. Beigi et al. showed the advantages of using titania as an orthopedic implant material and the surface modification strategies implemented in the study improved surface bioreactivity and osteogenesis for assisted bone tissue development. The cost-effective frugal method can provide a metallic nanofiber structure surface to be coated on multiple surfaces for varied biomedical applications. The proposed technique (combining materials engineering with stem cells) will open new doors to engineer advanced biomaterials with enhanced surface bioreactivity for improved biocompatibility in vitro and in vivo. The findings demonstrate beneficial effects of an experimental scaffold in the lab with potential for medical osseointegration as a BTE implant.
More information: Mohammad-Hossein Beigi et al. 3-D Titania Nanofiber-Like Webs Induced by Plasma Ionization: A New Direction for Bioreactivity and Osteoinductivity Enhancement of Biomaterials, Scientific Reports (2019). DOI: 10.1038/s41598-019-54533-z K.
Rezwan et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering, Biomaterials (2006). DOI: 10.1016/j.biomaterials.2006.01.039
Antonio Uccelli et al. Mesenchymal stem cells in health and disease, Nature Reviews Immunology (2008). DOI: 10.1038/nri2395
Journal information: Scientific Reports , Biomaterials , Nature Reviews Immunology
© 2019 Science X Network