This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Neurobiologists reveal secret of congenital central hypoventilation syndrome

In Greek mythology, a water nymph's curse forces a man to stay awake or suffocate. For a rare segment of the population today, the curse is all too real. Now, a team led by University of Connecticut researchers describes in Nature Communications how the curse works on molecules in the brain.
People with Ondine's curse, also known as congenital central hypoventilation syndrome, lack the ability to regulate breathing unconsciously. Such people must have a machine help them breathe when they sleep, or risk death. Poor sleep and less oxygen lead to health and learning problems, and people with the condition often die young.
The condition is also terribly stressful for the family of a person with Ondine's curse.
Dan Mulkey, a neurobiologist at UConn, describes how a colleague hitched a ride to a scientific conference with the family of a child who had the disorder. It was at night. They hit traffic. The baby would periodically fall asleep. The scientist sat in the backseat and every few minutes, he had to rouse the baby.
"If you have kids, you know that feeling. You wake them up because you want them to breathe," says Mulkey, referencing new parents' common anxieties about babies breathing. "But then they get cranky."
For families coping with Ondine's curse, a cranky baby is infinitely better than the alternative.
For most people, breathing is automatic. We can give it zero thought, and it just happens. But there's a great deal packed into that phrase "it just happens."
A small group of cells in the brainstem controls respiration. These cells are exquisitely attuned to levels of CO2 in our bloodstream. If CO2 levels increase, those cells increase our breathing rate to clear out CO2 and get more oxygen. But in people with Ondine's curse, those cells malfunction. When these people fall asleep, they rely on machines to keep them breathing.
Mulkey and Anastasios Tzingounis, another UConn neurobiologist, focus their research on that small group of cells in the brainstem. In people with Ondine's curse, it's thought that these CO2-attuned cells fail to develop, leading to the loss of the drive to breathe.
Now, Tzingounis, Mulkey, and a team of researchers led by UConn graduate student Jaseph Soto report that these CO2 sensing cells in the brainstem are remarkably sensitive to mutations in a potassium channel known as KCNQ2.
In particular, they show that a specific mutation in KCNQ2 results in breathing problems reminiscent of Ondine's curse.
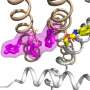
The KCNQ channels—there are five of them—work together in various combinations to move potassium ions in and out of cells. But in the brainstem cells that regulate breathing, KCNQ2 appears alone.
"In the area we study, because there are no other KCNQ channels, the mutation is free to run wild," Mulkey says.
By run wild, he means it has what is called a gain-of-function mutation. The channel is too active, allowing potassium to constantly flow out of the cell, which causes cells to go quiet. They stop signaling the body to breathe.
"When the KCNQ channels get that active, it's like a hole in the membrane" of the cell, Tzingounis says. "If they're super active, it keeps the brain very quiet."
Too quiet.
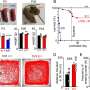
Tzingounis engineered mice to have KCNQ2 gain-of-function mutations, and with Soto's help found these mice have almost as much trouble breathing during times of low activity, when the mice should be sleeping, as people with Ondine's curse. In this case, the mice have the cells that drive breathing, but their activity is diminished by the KCNQ2 mutation.
People can also have this KCNQ2 gain-of-function mutation, and it is a common cause of developmental encephalopathy. They display diminished respiratory activity, similar to the mice. Interestingly, mutations in other KCNQ channels do not result in breathing problems, indicating brain cells that control breathing are very sensitive to disruption of KCNQ2 specifically.
Because KCNQ channels normally work together in the body, perhaps other KCNQ channels compensate for a slack KCNQ2, everywhere except in those special cells that stimulate breathing in the brainstem. Those brainstem breathing cells lack any other type of KCNQ channel and so are uniquely vulnerable.
More information: J. Soto-Perez et al, Phox2b-expressing neurons contribute to breathing problems in Kcnq2 loss- and gain-of-function encephalopathy models, Nature Communications (2023). DOI: 10.1038/s41467-023-43834-7