This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chromatin openness sheds new light on prostate cancer plasticity

Treatment resistance caused by cancer cell plasticity constitutes a major challenge in the treatment of prostate cancer. A recent study from the University of Eastern Finland Institute of Biomedicine published in Nucleic Acids Research, suggests that the SIX2 protein may be a possible factor underlying increased plasticity of prostate cancer cells and treatment resistance.
Prostate cancer is the most common cancer in men and the second most common cause of cancer mortality in Western countries. Prostate cancer growth is promoted by androgens and can be treated with androgen receptor inhibition therapies, especially in aggressive or advanced prostate cancer. However, cancer cells can develop resistance to these therapies, resulting in castration-resistant prostate cancer.
One mechanism underlying treatment resistance may be the plasticity of cancer cells: They can change their degree of differentiation and revert to a stem cell-like state, which helps them avoid the effects of hormonal therapies. However, factors contributing to cell plasticity and the development of treatment resistance remain unclear.
"It is important to identify the key factors contributing to treatment resistance in prostate cancer and how cancer cells change their degree of differentiation to find new targets for therapies. This could even lead to the discovery of a cure for these currently lethal types of cancer," Academy Research Fellow, Adjunct Professor (Docent) Kirsi Ketola of the University of Eastern Finland says.
Inhibition of the androgen receptor opens up new genomic regions
The new study by the Ketola Lab explored new potential factors contributing to treatment resistance in prostate cancer.
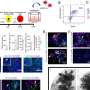
In cells, DNA is packed into chromatin. In regions where gene expression is active, this packing is looser, meaning that chromatin is more open. The Ketola Lab studied chromatin openness in androgen-dependent prostate cancer cells, which were treated with enzalutamide, an inhibitor of the androgen receptor used for treating prostate cancer.
The researchers found that following exposure to enzalutamide, the number of new opened chromatin sites was greater than that of new closed chromatin sites. These new opened sites occurred especially in DNA regions containing binding site of the SIX2 protein. The increased activity of the SIX2 protein may contribute to the increased plasticity of cells following drug therapy.
In other words, inhibiting the function of the androgen receptor alters the regulation of genes within cells, allowing for the expression of genes that are normally silenced and the alteration of the cell state.
SIX2 is necessary for embryogenesis, but increases cell plasticity and malignancy in prostate cancer cells
The SIX2 protein is normally active during embryogenesis, where it maintains cells as undifferentiated stem cells, preserving their ability to differentiate.
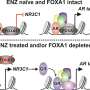
The study found that the SIX2 protein can regulate the degree of differentiation of even prostate cancer cells that do not have an androgen receptor. The activity of the SIX2 gene increased in cancer cells after exposure to enzalutamide. In particular, the expression of the SIX2 protein has increased in cancer cells that do not express the androgen receptor.
"Silencing of the SIX2 gene, on the other hand, significantly reduced the malignancy of cancer cells that are resistant to hormonal therapies," Doctoral Researcher Noora Leppänen of the University of Eastern Finland notes.
The stem cell-like state of cancer cells that do not express the androgen receptor, as well as their ability to migrate, invade and metastasize, were significantly reduced following the silencing of the SIX2 gene. Reduced cell division and cancer spread were also observed in experiments conducted on zebrafish.
Therefore, inhibiting the activity of the SIX2 protein could be a potential target for drug development to treat or prevent the development of metastatic, hormone therapy-resistant types of cancer.
More information: Noora Leppänen et al, SIX2 promotes cell plasticity via Wnt/β-catenin signalling in androgen receptor independent prostate cancer, Nucleic Acids Research (2024). DOI: 10.1093/nar/gkae206