Abstract
Melanoma incidence is increasing rapidly worldwide among white-skinned populations. Earlier diagnosis is the principal factor that can improve prognosis. Defining high-risk populations using risk prediction models may help targeted screening and early detection approaches. In this systematic review, we searched Medline, EMBASE, and the Cochrane Library for primary research studies reporting or validating models to predict risk of developing cutaneous melanoma. A total of 4,141 articles were identified from the literature search and six through citation searching. Twenty-five risk models were included. Between them, the models considered 144 possible risk factors, including 18 measures of number of nevi and 26 of sun/UV exposure. Those most frequently included in final risk models were number of nevi, presence of freckles, history of sunburn, hair color, and skin color. Despite the different factors included and different cutoff values for sensitivity and specificity, almost all models yielded sensitivities and specificities that fit along a summary ROC with area under the ROC (AUROC) of 0.755, suggesting that most models had similar discrimination. Only two models have been validated in separate populations and both also showed good discrimination with AUROC values of 0.79 (0.70–0.86) and 0.70 (0.64–0.77). Further research should focus on validating existing models rather than developing new ones. Cancer Epidemiol Biomarkers Prev; 23(8); 1450–63. ©2014 AACR.
Introduction
Melanoma is one of the fastest growing cancers worldwide: Age-adjusted incidence rates have been increasing in most of the fair-skinned populations in recent decades; and 160,000 new cases are diagnosed annually worldwide (1–5). As earlier diagnosis is the principal factor that can improve the prognosis of patients with melanoma (6), there is considerable interest in the development of screening programs. The SCREEN project in northern Germany suggested that population screening may have a substantial impact on melanoma incidence and 5-year mortality (7, 8) leading to the implementation of a national statutory skin cancer early detection program in Germany in 2008. However, such mass screening is not currently recommended by the U.S. Preventive Services Task Force (9) or in other countries. Modeling studies suggest that selective, targeted screening might be a more cost-effective strategy (10, 11). Such a stratified approach is currently recommended by the Royal Australian College of General Practitioners. Australian primary care physicians are advised to perform skin examinations every 3 to 12 months in people with multiple atypical or dysplastic nevi and a history of melanoma or a first-degree relative with melanoma (12). This approach is also being considered by the Department of Health in the United Kingdom.
The aims of such targeted screening programs are to identify people at higher risk of melanoma and to offer them preventive advice about sun protection and skin awareness and early consultation or surveillance (13–15). The identification of people at higher risk may be improved by the use of risk prediction models. Several risk models have been developed but their strengths, weaknesses, and relative performance are uncertain. We report a systematic review and comparison of risk prediction models for melanoma.
Materials and Methods
Search strategy
An electronic literature search of Medline, EMBASE, and the Cochrane Library up to August 2013 was performed using a combination of subject headings and free text incorporating “melanoma,” “risk/risk factor/risk assessment/chance,” and “prediction/model/score” (see Supplementary File S1 for complete search strategy). We then manually screened the reference lists of all included articles.
Study selection
Studies were included if they fulfilled all of the following criteria: (i) are published as a primary research article in a peer-reviewed journal; (ii) identify risk factors for developing melanoma at the level of the individual; (iii) provide a measure of relative or absolute risk using a combination of risk factors that allows identification of people at higher risk of melanoma; (iv) use a statistical method to develop the final risk model; and (v) are applicable to the general population. As the focus of the review is to summarize the risk prediction models for incident melanoma, studies developing models for the risk of recurrence and prognostic models were excluded. Studies including only highly selected groups, for example, immunosuppressed patients or those with a previous history of cancer, and conference proceedings were also excluded. The decision to only include articles which use a statistical method to develop the final risk model was made to differentiate between those studies which had set out to develop a risk model, using either a stepwise method or maximization of sensitivity and specificity to select the variables for the final model, from the large number of variable-finding studies which provide tables with odds ratios or risk ratios adjusted simultaneously for all considered variables but do not attempt to generate or test a risk model.
One reviewer (J.A. Usher-Smith) performed the search and screened the titles and abstracts to exclude articles that were clearly not relevant. A second reviewer (F.M. Walter) independently assessed a random selection of 5% of the articles excluded at that stage. For articles where a definite decision to reject could not be made based on title and abstract alone, the full text was examined. At least 2 reviewers (J.A. Usher-Smith and J. Emery/A.P. Kassianos/F.M. Walter) independently assessed all full-text articles, and those deemed not to meet inclusion criteria by both researchers were excluded. Articles for which it was unclear whether or not the inclusion criteria were met were discussed at consensus meetings including all researchers. Articles written in languages other than English were translated into English for assessment and subsequent data extraction.
Data extraction and synthesis
Data were extracted independently by at least 2 researchers (J.A. Usher-Smith and J. Emery/A.P. Kassianos/F.M. Walter) using a standardized form to minimize bias. The form included details on: (i) the development of the model, including the study design, selection of participants, the variables considered for inclusion in the model, and how they were selected; (ii) the risk model itself, including the variables included, the method of administration, and whether it requires physician input or population training; (iii) the performance of the risk model in the development population, including measures of discrimination, accuracy, calibration, and use; and (iv) validation studies of the risk model and data collection tool, including the study design and performance of the risk model.
For studies which reported the stepwise performance of models, only the model with the best performance was included. For studies which included multiple different models, for example separate models for men and women or for self-assessment and physician assessment, all were included separately. One article (16) reported models for 2 different age groups in addition to the cohort as a whole. In this case, only the model for the entire cohort was included.
During the data extraction, risk factors were grouped into the following categories: personal characteristics, genetic factors, female hormonal factors, access to specialist skin care, personal medical history, family history, hair color, eye color, skin type (Fitzpatrick), skin color, skin response to sun, history of sunburn, use of sun protection, number of nevi, number of atypical or dysplastic nevi, freckles, congenital nevi, other skin findings, new or changing nevi, sun/UV exposure (including sun bed use), and UV skin damage. Separate categories were included for skin color, skin response to the sun, and skin type (Fitzpatrick), which includes both skin color and skin response to the sun (17). If articles used the term “skin type” but then defined that by the skin response to the sun, this was extracted under skin response to the sun.
Information concerning whether the risk models required physician input or could be performed without involvement of a healthcare professional was also extracted. Risk models were classified as requiring physician input if they included any of the following factors: dysplastic or atypical nevi, actinic lentigines, total body nevus count, genetic analysis requiring samples, or specialized equipment such as dermoscopy or colorimetry. Nevus density, as discussed by Marrett and colleagues (18), was not considered to require physician input as participants were provided with images representing a range of nevus density and counting of individual nevi was not required.
Reported measures of discrimination, accuracy, calibration, and use were used to compare the performance of risk models. The sensitivity and specificity of different models were also compared graphically by plotting a summary ROC curve using the Moses–Littenberg method (19, 20) in RevMan version 5.2 and the summary AUROC calculated in Meta-DiSc version 1.4 using Moses' constant for linear models to fit the summary ROC curve.
Results
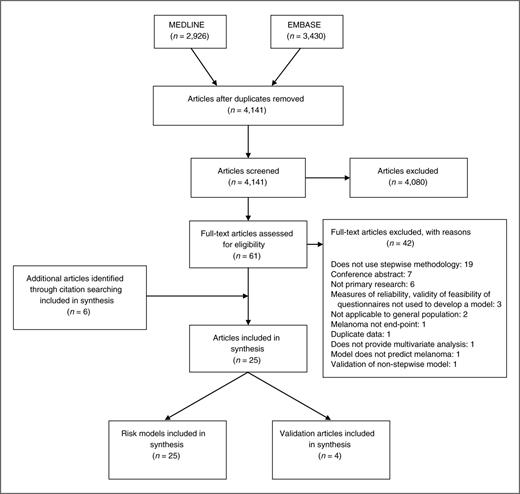
After duplicates were removed, the search identified 4,141 articles. 4,080 were excluded at title and abstract level. A further 42 were excluded after full-text assessment by at least 2 authors (J.A. Usher-Smith and J. Emery/A.P. Kassianos/F.M. Walter). There was complete agreement among researchers throughout the screening process, and the most common reasons for exclusion were that the articles did not use a statistical method to develop the final risk model, were conference abstracts, or not primary research. Two well-cited models, excluded because they were not developed using a statistical method, are those by Mar (21) and Glanz (22). Mar and colleagues selected risk factors and estimated RRs for risk factor combinations from existing large meta-analyses (23–25) and data from the Victorian Cancer Registry. Glanz developed the BRAT (Brief skin cancer Risk Assessment Tool) through critically reviewing published literature on risk factors and their self-assessment and then piloted the questionnaire on a convenience sample of people at varying levels of risk to estimate the range of scores and test–retest reliability of the tool. Neither tested the performance of the models in any populations with melanoma.
Six further articles were identified through citation searching giving 25 articles for inclusion. Of these, 4 provided validation of other models and 4 included more than one risk model. This review, therefore, describes 25 risk prediction models (Fig. 1).
A summary of these 25 models, along with measures of performance in the development population and notable strengths and weaknesses, is given in Table 1. Fifteen require physician input whereas 10 can be performed by self-assessment. Discriminatory performance was provided for 14. Most had values for the area under the ROC (AUROC) between 0.7 and 0.8 with little difference between those suitable for self-assessment and those requiring a health care professional. Poorer discrimination was seen in those models including only skin color and skin type (0.54; ref. 26), age, sex, cutaneous melanin, and genotyping (0.65; ref. 27) and in the only model developed in a cohort study to provide a measure of performance (0.62; ref. 28). The highest discrimination was for a model including a suspicious melanocytic lesion on dermoscopy (0.86; ref. 29) with a second model developed from a small case–control study in Brazil where there were more cases than controls also reporting high discrimination (0.85; ref. 30).
Summary of risk prediction models
| . | Components of score . | Model performance in development population . | General comments . | ||||
|---|---|---|---|---|---|---|---|
| Author . | Factors included in score . | Physician input? . | Discrimination . | Calibration . | Accuracy . | Strengths . | Limitations . |
| Augustsson (46) | Skin type, hair color, eye color, total body nevus ≥ 2 mm count, number of dysplastic nevi | Yes | 1) Reproducible as relies on observation rather than recall | 1) Developed from survivors of melanoma so may be biased towards less poor outcomes and lower stages | |||
| 2) Only applicable to people 30–50 years of age | |||||||
| Bakos et al. (30) | Hair color; presence of freckles; sunburns in all life; skin color; eye color | No | AUROC 0.85 (0.77–0.91) | 1) Good discrimination | 1) Developed from population with limited range of skin phototypes | ||
| Barbini et al. (26) | Skin color using colorimeter and skin type | Yes | AUROC 0.54 | Sens 86; Spec 45; PPV 0.3; NPV 0.92 | 1) Very complicated to calculate | ||
| 2) Poor discrimination with two thirds of subjects misclassified as high risk | |||||||
| Cho et al. (28) | Gender; age; family history of melanoma; history of severe sunburn; number of nevi > 3 mm on arms or lower legs; hair color | No | AUROC 0.62 (0.58–0.65) | χ2 goodness-of-fit 9.28; P = 0.41 | 1) Based on large cohort study with 16 years follow-up | 1) Based on predominantly female white health professionals only; | |
| 2) Would require a computer or expert to calculate risk using regression coefficients | |||||||
| Dwyer et al. (27) | Age; sex; cutaneous melanin; MC1R genotype | Yes | AUROC 0.65 | 1) Requires DNA sample; | |||
| 2) Only obtained genetic information from 67% of participants | |||||||
| English and Armstrong (35) | Number of raised nevi on the arms; age on arrival in Australia; history of non-melanocytic skin cancer; mean time spent outdoors in summer from the age of 10 to 24; family history of melanoma | No | Sens 54; Spec 84 | 1) High specificity | 1) One variable not transferable outside Australia and number of hours spent outdoors difficult to estimate | ||
| 2) Initially developed in 400 case–control pairs then refined in separate 111 pairs | |||||||
| 3) Developed from large study with population = based controls | |||||||
| Fears et al. (men; ref. 47) | Skin color; number of moles < 5 mm; freckling; number of moles ≥ 5 mm; severe solar skin damage | Yes | AUROC 0.7–0.8a | 1) Simple and quick with only 2 questions and examination of back | 1) Not applicable to people with prior melanoma or non-melanoma skin cancer or 1st degree relative with melanoma | ||
| Fears et al. (women; ref. 47) | Skin color; number of moles < 5 mm; freckling; tanning ability; number of moles ≥ 5 mm; severe solar skin damage | Yes | AUROC 0.7–0.8a | 1) Simple and quick with only 2 questions and examination of back | 1) Not applicable to people with prior melanoma or non-melanoma skin cancer or 1st degree relative with melanoma | ||
| Fortes et al. (33) | Hair color; skin type; presence of freckles; number of common nevi on the whole body; sunburn as a child | Yes | AUROC 0.79 (0.75–0.82) | Risk cutoff ≥ 3; Sens 88.6; Spec 51.4b | 1) Externally validated | 1) Developed from study with hospital based controls | |
| 2) Good discrimination and high sensitivity | 2) Potential for recall bias with sunburn as a child | ||||||
| Garbe et al. (48) | Total number of nevi ≥ 2 mm, total number of atypical nevi, actinic lentigines, occupational sun exposure and skin response to sun | Yes | 1) Requires physician whole-body examination by dermatologist so not feasible for primary care | ||||
| Garbe et al. (49) | Number of nevi ≥ 2 mm; presence of actinic lentigines; number of atypical melanocytic nevi; skin type; growth of any existing melanocytic nevi, hair color | Yes | 1) Reproducible as relies on observation rather than recall | 1) Hospital-based controls from dermatology department | |||
| 2) Requires physician whole body examination by dermatologist so not feasible for primary care | |||||||
| Goldberg et al. (32) | History of previous melanoma, age > 50, does not see regular dermatologist, changing mole, gender | No | 1) Reproducible as relies on observation rather than recall | 1) Based on study with no follow-up of patients and no histological diagnosis | |||
| 2) Risk of bias as developed from self-selected population | |||||||
| 3) Questionable relevance of absent dermatologist outside USA | |||||||
| Guther et al. (29) | Age; hair color; past history of melanoma; suspicious melanocytic lesion on dermatoscopy | Yes | AUROC 0.86 | χ2 Likelihood ratio: P < 0.0001 | Sens 92.3 | 1) High discrimination and calibration | 1) Requires dermatoscopic examination |
| 2) Reproducible as relies on observation rather than recall | 2) Risk of bias as developed from self-selected population attending dermatologist | ||||||
| Harbaue et al. (physician assessment; ref. 39) | Skin type; UV damage to skin; number of nevi | Yes | AUROC 0.77 (0.73–0.83) | Sens 42 (95% CI, 33–52); Spec 90 | 1) Simple | 1) Would require a computer to implement risk model | |
| 2) Good discrimination | 2) Unclear how UV score was calculated | ||||||
| 3) Potential for bias as developed from population with controls from private GP or dermatology | |||||||
| Harbauer 2003 (self- assessment) (39) | Skin type; UV damage to skin; number of nevi | No | AUROC 0.73 (0.6–0.77) | Sens 39 (95% CI, 31–48); Spec 90 | 1) Simple | 1) Would require a computer to implement risk model | |
| 2) Good discrimination | 2) Potential for bias as developed from population with controls from private GP or dermatology | ||||||
| Landi et al. (50) | Presence of dysplastic nevi; skin color; propensity to tan; eye color | Yes | 1) Reproducible as relies on observation rather than recall | 1) Developed from population with most controls friends or family members of cases so potential for bias | |||
| MacKie et al. (51) | Gender, total number of nevi ≥ 2 mm diameter; freckling tendency; number of clinically atypical nevi; number of episodes of severe sunburn at any time in life | Yes | 1) Relatively simple to use flowchart | 1) Potential for recall bias with number of episodes of severe sunburn at any time in life | |||
| Marrett et al. (18) | Hair color; skin reaction to repeated sun exposure; freckle density; nevus density | No | Sens 40; Spec 89 | 1) Limited opportunity for recall bias | 1) Not applicable to patients with previous melanoma | ||
| Neilsen et al. (16) | Family history, number of nevi ≥ 3 mm on left arm, hair color, time spent on sunbathing vacations | No | 1) Developed from population-based cohort study | 1) Only applies to women | |||
| 2) Based on small number of cases as relatively short period of follow-up | |||||||
| Quereux et al. (1; ref. 31) | Sunburn in childhood; family history of melanoma; number of nevi on arms; density of freckles; skin type; total sun exposure | No | AUROC 0.70 | Risk cutoff: 24; Sens 60.2 ± 2.8; Spec 71.1 ± 1.2 | 1) Good discrimination | 1) Total sun exposure difficult to calculate | |
| 2) Potential for recall bias with sunburn in childhood | |||||||
| Quereux et al. (2; ref. 31) | Sex; age; skin type; freckles; number of nevi on arms; severe blistering sunburn as a child; life in a country at low altitude; melanoma in a first-degree relative | No | AUROC 0.73 | Hosmer–Lemeshow statistic P = 0.43d | Risk cutoff: 13; Sens 64.9 ± 3.4; Spec 68.4 ± 1.3 | 1) Good discrimination | 1) Potential for recall bias with sunburn as a child |
| Quereux et al. (3; ref. 31) | SAMScorec: phototype I or II; freckling tendency; >20 nevi on both arms; severe sunburn during childhood or teenage years; life in a country at low altitude; a history of previous melanoma; history of melanoma in a first-degree relative | No | AUROC 0.71 | Sens 63.2 ± 3.6; Spec 68.8 ± 1.2 | 1) Good discrimination | 1) Combinatorial analysis quite complicated | |
| 2) Potential for recall bias with sunburn as a child | |||||||
| Weiss et al. (52) | Total number of nevi > 2 mm over whole body, hair color, occupational sun exposure, and skin response to sun | Yes | 1) Unclear description of variables included | ||||
| Williams et al. (34) | Age; sex; number of severe sunburns aged 2–18; hair color age 15; density of freckles on arms before aged 20; number of raised moles on both arms; prior non-melanoma skin cancer | No | AUROC 0.77 (0.73–0.81) | Cutoff: 25; Sens 61; Spec 80b | 1) Good discrimination | 1) Not yet validated for self-completion | |
| AUROC 0.70 (0.64–0.77)b | Cutoff: 28; Sens 50; Spec 85b | 2) Validated on separate group | 2) Only applicable for ages 35–74 | ||||
| Cutoff: 30; Sens 42; Spec 90b | 3) Only counting raised moles distinguishes from freckles | 3) Potential recall bias for number of freckles before age 20 | |||||
| Cutoff: 34; Sens 29; Spec 95b | |||||||
| Zaridze et al. (53) | Presence of freckles on arms; number of raised moles on arms and moles on body > 6 mm; skin color; eye color; frequency of sunbathing during lifetime | No | 1) Only counting raised moles distinguishes from freckles | 1) Poor description of variables included | |||
| 2) Potential for recall bias with frequency of sunbathing during lifetime | |||||||
| . | Components of score . | Model performance in development population . | General comments . | ||||
|---|---|---|---|---|---|---|---|
| Author . | Factors included in score . | Physician input? . | Discrimination . | Calibration . | Accuracy . | Strengths . | Limitations . |
| Augustsson (46) | Skin type, hair color, eye color, total body nevus ≥ 2 mm count, number of dysplastic nevi | Yes | 1) Reproducible as relies on observation rather than recall | 1) Developed from survivors of melanoma so may be biased towards less poor outcomes and lower stages | |||
| 2) Only applicable to people 30–50 years of age | |||||||
| Bakos et al. (30) | Hair color; presence of freckles; sunburns in all life; skin color; eye color | No | AUROC 0.85 (0.77–0.91) | 1) Good discrimination | 1) Developed from population with limited range of skin phototypes | ||
| Barbini et al. (26) | Skin color using colorimeter and skin type | Yes | AUROC 0.54 | Sens 86; Spec 45; PPV 0.3; NPV 0.92 | 1) Very complicated to calculate | ||
| 2) Poor discrimination with two thirds of subjects misclassified as high risk | |||||||
| Cho et al. (28) | Gender; age; family history of melanoma; history of severe sunburn; number of nevi > 3 mm on arms or lower legs; hair color | No | AUROC 0.62 (0.58–0.65) | χ2 goodness-of-fit 9.28; P = 0.41 | 1) Based on large cohort study with 16 years follow-up | 1) Based on predominantly female white health professionals only; | |
| 2) Would require a computer or expert to calculate risk using regression coefficients | |||||||
| Dwyer et al. (27) | Age; sex; cutaneous melanin; MC1R genotype | Yes | AUROC 0.65 | 1) Requires DNA sample; | |||
| 2) Only obtained genetic information from 67% of participants | |||||||
| English and Armstrong (35) | Number of raised nevi on the arms; age on arrival in Australia; history of non-melanocytic skin cancer; mean time spent outdoors in summer from the age of 10 to 24; family history of melanoma | No | Sens 54; Spec 84 | 1) High specificity | 1) One variable not transferable outside Australia and number of hours spent outdoors difficult to estimate | ||
| 2) Initially developed in 400 case–control pairs then refined in separate 111 pairs | |||||||
| 3) Developed from large study with population = based controls | |||||||
| Fears et al. (men; ref. 47) | Skin color; number of moles < 5 mm; freckling; number of moles ≥ 5 mm; severe solar skin damage | Yes | AUROC 0.7–0.8a | 1) Simple and quick with only 2 questions and examination of back | 1) Not applicable to people with prior melanoma or non-melanoma skin cancer or 1st degree relative with melanoma | ||
| Fears et al. (women; ref. 47) | Skin color; number of moles < 5 mm; freckling; tanning ability; number of moles ≥ 5 mm; severe solar skin damage | Yes | AUROC 0.7–0.8a | 1) Simple and quick with only 2 questions and examination of back | 1) Not applicable to people with prior melanoma or non-melanoma skin cancer or 1st degree relative with melanoma | ||
| Fortes et al. (33) | Hair color; skin type; presence of freckles; number of common nevi on the whole body; sunburn as a child | Yes | AUROC 0.79 (0.75–0.82) | Risk cutoff ≥ 3; Sens 88.6; Spec 51.4b | 1) Externally validated | 1) Developed from study with hospital based controls | |
| 2) Good discrimination and high sensitivity | 2) Potential for recall bias with sunburn as a child | ||||||
| Garbe et al. (48) | Total number of nevi ≥ 2 mm, total number of atypical nevi, actinic lentigines, occupational sun exposure and skin response to sun | Yes | 1) Requires physician whole-body examination by dermatologist so not feasible for primary care | ||||
| Garbe et al. (49) | Number of nevi ≥ 2 mm; presence of actinic lentigines; number of atypical melanocytic nevi; skin type; growth of any existing melanocytic nevi, hair color | Yes | 1) Reproducible as relies on observation rather than recall | 1) Hospital-based controls from dermatology department | |||
| 2) Requires physician whole body examination by dermatologist so not feasible for primary care | |||||||
| Goldberg et al. (32) | History of previous melanoma, age > 50, does not see regular dermatologist, changing mole, gender | No | 1) Reproducible as relies on observation rather than recall | 1) Based on study with no follow-up of patients and no histological diagnosis | |||
| 2) Risk of bias as developed from self-selected population | |||||||
| 3) Questionable relevance of absent dermatologist outside USA | |||||||
| Guther et al. (29) | Age; hair color; past history of melanoma; suspicious melanocytic lesion on dermatoscopy | Yes | AUROC 0.86 | χ2 Likelihood ratio: P < 0.0001 | Sens 92.3 | 1) High discrimination and calibration | 1) Requires dermatoscopic examination |
| 2) Reproducible as relies on observation rather than recall | 2) Risk of bias as developed from self-selected population attending dermatologist | ||||||
| Harbaue et al. (physician assessment; ref. 39) | Skin type; UV damage to skin; number of nevi | Yes | AUROC 0.77 (0.73–0.83) | Sens 42 (95% CI, 33–52); Spec 90 | 1) Simple | 1) Would require a computer to implement risk model | |
| 2) Good discrimination | 2) Unclear how UV score was calculated | ||||||
| 3) Potential for bias as developed from population with controls from private GP or dermatology | |||||||
| Harbauer 2003 (self- assessment) (39) | Skin type; UV damage to skin; number of nevi | No | AUROC 0.73 (0.6–0.77) | Sens 39 (95% CI, 31–48); Spec 90 | 1) Simple | 1) Would require a computer to implement risk model | |
| 2) Good discrimination | 2) Potential for bias as developed from population with controls from private GP or dermatology | ||||||
| Landi et al. (50) | Presence of dysplastic nevi; skin color; propensity to tan; eye color | Yes | 1) Reproducible as relies on observation rather than recall | 1) Developed from population with most controls friends or family members of cases so potential for bias | |||
| MacKie et al. (51) | Gender, total number of nevi ≥ 2 mm diameter; freckling tendency; number of clinically atypical nevi; number of episodes of severe sunburn at any time in life | Yes | 1) Relatively simple to use flowchart | 1) Potential for recall bias with number of episodes of severe sunburn at any time in life | |||
| Marrett et al. (18) | Hair color; skin reaction to repeated sun exposure; freckle density; nevus density | No | Sens 40; Spec 89 | 1) Limited opportunity for recall bias | 1) Not applicable to patients with previous melanoma | ||
| Neilsen et al. (16) | Family history, number of nevi ≥ 3 mm on left arm, hair color, time spent on sunbathing vacations | No | 1) Developed from population-based cohort study | 1) Only applies to women | |||
| 2) Based on small number of cases as relatively short period of follow-up | |||||||
| Quereux et al. (1; ref. 31) | Sunburn in childhood; family history of melanoma; number of nevi on arms; density of freckles; skin type; total sun exposure | No | AUROC 0.70 | Risk cutoff: 24; Sens 60.2 ± 2.8; Spec 71.1 ± 1.2 | 1) Good discrimination | 1) Total sun exposure difficult to calculate | |
| 2) Potential for recall bias with sunburn in childhood | |||||||
| Quereux et al. (2; ref. 31) | Sex; age; skin type; freckles; number of nevi on arms; severe blistering sunburn as a child; life in a country at low altitude; melanoma in a first-degree relative | No | AUROC 0.73 | Hosmer–Lemeshow statistic P = 0.43d | Risk cutoff: 13; Sens 64.9 ± 3.4; Spec 68.4 ± 1.3 | 1) Good discrimination | 1) Potential for recall bias with sunburn as a child |
| Quereux et al. (3; ref. 31) | SAMScorec: phototype I or II; freckling tendency; >20 nevi on both arms; severe sunburn during childhood or teenage years; life in a country at low altitude; a history of previous melanoma; history of melanoma in a first-degree relative | No | AUROC 0.71 | Sens 63.2 ± 3.6; Spec 68.8 ± 1.2 | 1) Good discrimination | 1) Combinatorial analysis quite complicated | |
| 2) Potential for recall bias with sunburn as a child | |||||||
| Weiss et al. (52) | Total number of nevi > 2 mm over whole body, hair color, occupational sun exposure, and skin response to sun | Yes | 1) Unclear description of variables included | ||||
| Williams et al. (34) | Age; sex; number of severe sunburns aged 2–18; hair color age 15; density of freckles on arms before aged 20; number of raised moles on both arms; prior non-melanoma skin cancer | No | AUROC 0.77 (0.73–0.81) | Cutoff: 25; Sens 61; Spec 80b | 1) Good discrimination | 1) Not yet validated for self-completion | |
| AUROC 0.70 (0.64–0.77)b | Cutoff: 28; Sens 50; Spec 85b | 2) Validated on separate group | 2) Only applicable for ages 35–74 | ||||
| Cutoff: 30; Sens 42; Spec 90b | 3) Only counting raised moles distinguishes from freckles | 3) Potential recall bias for number of freckles before age 20 | |||||
| Cutoff: 34; Sens 29; Spec 95b | |||||||
| Zaridze et al. (53) | Presence of freckles on arms; number of raised moles on arms and moles on body > 6 mm; skin color; eye color; frequency of sunbathing during lifetime | No | 1) Only counting raised moles distinguishes from freckles | 1) Poor description of variables included | |||
| 2) Potential for recall bias with frequency of sunbathing during lifetime | |||||||
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
aUses US Surveillance, Epidemiology, and End Results Program (SEER) and hypothetical cohort rather than testing on this case–control study population.
bFrom validation study in different population to development of the model.
cAccording to the SAMScore, a patient is considered at risk of melanoma if at least 1 of these 3 criteria is verified: (i) presence of at least 3 risk factors among the 7 following risk factors: phototype I or II, freckling tendency, number of melanocytic nevi >20 on both arms, severe sunburn during childhood or teenage years, life in a country at low latitude, a history of previous melanoma, a history of melanoma in a first-degree relative. (ii) A subject younger than 60 years and a number of melanocytic nevi >20 on both arms. (iii) A subject aged 60 years old or over and a freckling tendency.
dThe Hosmer–Lemeshow statistic assesses whether or not the observed event rates match expected event rates in subgroups of deciles of fitted risk values. A nonsignificant P value indicates a well-calibrated model.
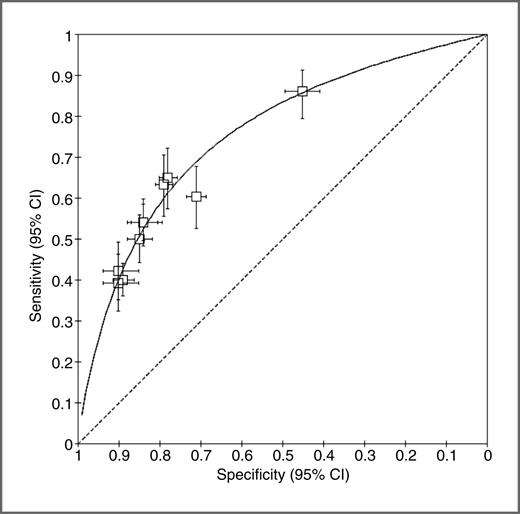
A measure of accuracy was provided in 10 studies. The sensitivity and specificity varied between them, but a summary ROC curve (Fig. 2) shows that they all lie very close to the curve. This shows that despite all the heterogeneity in model development and risk factors, there is very little heterogeneity in the predictive ability of the models with the variation in sensitivity and specificity likely a reflection of the cutoff values chosen in different studies. The AUROC of this summary curve is 0.755.
Summary ROC curve for the 10 models that provide values for sensitivity and specificity. Each data point represents the sensitivity and specificity for a single threshold of a risk model with horizontal and vertical bars indicating 95% confidence intervals (CI). The AUROC of this summary curve is 0.755.
Summary ROC curve for the 10 models that provide values for sensitivity and specificity. Each data point represents the sensitivity and specificity for a single threshold of a risk model with horizontal and vertical bars indicating 95% confidence intervals (CI). The AUROC of this summary curve is 0.755.
Only 3 models had reported measures of calibration (28, 29, 31). All 3 showed good calibration but all had been tested in the development population where calibration would be expected to be high.
Further details of the development of each model are given in Supplementary Tables S1 and S2 for case–control and cohort studies, respectively. Twenty-one were case–control studies and 4 cohort studies. Overall, the reporting of the studies was variable. Of the 21 case–control studies, the method of selecting the variables for consideration was given in only 11, of which for 8 the method was a literature review, and the predictor variables and outcomes were evaluated in a blinded fashion in only 4. Cases were selected from either cancer registries or dermatology clinics and all required histologic confirmation of diagnosis. Controls were selected from hospital clinics in 7 studies, the general population in 5, dermatology clinics in 4, and primary care in 3. Most controls were matched by age and gender with a mean age of 43 to 57 years. Of the 4 cohort studies, only Neilsen and colleagues (16) provided any detail of the method of selecting the variables, none were evaluated in a blinded fashion, and Goldberg (32) did not require a histologic diagnosis. Neilsen and colleagues (16) included only female participants, while the study of Cho and colleagues (28) was also heavily female dominated.
Table 2 shows additional details of those models in which either the model itself or the method of data collection used for the model has been validated or in which efficiency has been estimated. Only one model, Fortes and colleagues (33), has been validated in an external population and one, Williams and colleagues (34), in a separate subgroup of the original study population. English and colleagues (35) also divided their initial study population into 2 but they used the second subpopulation to further refine the model developed in the first subpopulation rather than validate the performance of the model in a separate population.
Details of validation studies
| Risk model . | Study . | Country, year . | Study design . | Data collection method . | Selection of cases . | Selection of controls . | Number of cases:controls (participation rate, %) . | Discrimination . | Accuracy . | Utility . |
|---|---|---|---|---|---|---|---|---|---|---|
| Fortes et al. (33) | Fortes et al. (33) | Brazil, 2005–8 | Case–control | Interview-administered questionnaire and examination | Caucasian individuals with histologically confirmed primary melanoma, >18, and resident in study area | Caucasian patients from general wards without a personal history of skin cancer matched by age and sex | 64 (97%):53 (100%) | AUROC 0.79 (0.70–0.86) | Cutoff level ≥ 3; Sens 79.6; Spec 60 | |
| Williams et al. (34) | Williams et al. (34) | USA, 1997 | Case–control (subset of original study) | Telephone survey | Patients with primary invasive cutaneous melanoma from surveillance epidemiology and cancer register | Random digit dialing | 25% of 386 (80%): 727 (63%)a | AUROC 0.70 (0.64–0.77) | Cutoff: 25; Sens 61; Spec 80 Cutoff: 28; Sens 50; Spec 85 Cutoff: 30; Sens 42; Spec 90 Cutoff: 34; Sens 29; Spec 95b | |
| MacKie et al. (51) | Jackson et al. (54) | UK, 1995 | Cohort | Self-completion of questionnaire and examination | Consecutive patients > 16 visiting their doctor | 388 (26%)c | Agreement of self-report and clinical examination: κ 0.43–0.67 | |||
| Neilsen et al. (16) | Westerdalh et al. (55) | Sweden, 1990–4 | Cohort | Postal questionnaire | Random sampling of women who had responded to initial questionnaire 1–3 years previously | 670 (84%) | Test–retest reliability of questionnaire: κ 0.54–0.83 | |||
| Quereux et al. (1, 2, 3; ref. 31) | Quereux et al. (56) | France, 2006–7 | Cohort | Self-completion of questionnaire and examination | Consecutive patients 18–70 years visiting their doctor | 1,358 (100%)d | Agreement of self-report and clinical examination:% correct answers 79.9–98.1 | |||
| Quereux et al. (3; ref. 31) | Quereux et al. (57) | France, 2009 | Cohort | Self-completion of questionnaire and examination of patients at high risk | Consecutive patients > 18 visiting their doctor | 1,039 (43%)e | Efficiency 11.54 (P = 0.0016)f | |||
| Risk model . | Study . | Country, year . | Study design . | Data collection method . | Selection of cases . | Selection of controls . | Number of cases:controls (participation rate, %) . | Discrimination . | Accuracy . | Utility . |
|---|---|---|---|---|---|---|---|---|---|---|
| Fortes et al. (33) | Fortes et al. (33) | Brazil, 2005–8 | Case–control | Interview-administered questionnaire and examination | Caucasian individuals with histologically confirmed primary melanoma, >18, and resident in study area | Caucasian patients from general wards without a personal history of skin cancer matched by age and sex | 64 (97%):53 (100%) | AUROC 0.79 (0.70–0.86) | Cutoff level ≥ 3; Sens 79.6; Spec 60 | |
| Williams et al. (34) | Williams et al. (34) | USA, 1997 | Case–control (subset of original study) | Telephone survey | Patients with primary invasive cutaneous melanoma from surveillance epidemiology and cancer register | Random digit dialing | 25% of 386 (80%): 727 (63%)a | AUROC 0.70 (0.64–0.77) | Cutoff: 25; Sens 61; Spec 80 Cutoff: 28; Sens 50; Spec 85 Cutoff: 30; Sens 42; Spec 90 Cutoff: 34; Sens 29; Spec 95b | |
| MacKie et al. (51) | Jackson et al. (54) | UK, 1995 | Cohort | Self-completion of questionnaire and examination | Consecutive patients > 16 visiting their doctor | 388 (26%)c | Agreement of self-report and clinical examination: κ 0.43–0.67 | |||
| Neilsen et al. (16) | Westerdalh et al. (55) | Sweden, 1990–4 | Cohort | Postal questionnaire | Random sampling of women who had responded to initial questionnaire 1–3 years previously | 670 (84%) | Test–retest reliability of questionnaire: κ 0.54–0.83 | |||
| Quereux et al. (1, 2, 3; ref. 31) | Quereux et al. (56) | France, 2006–7 | Cohort | Self-completion of questionnaire and examination | Consecutive patients 18–70 years visiting their doctor | 1,358 (100%)d | Agreement of self-report and clinical examination:% correct answers 79.9–98.1 | |||
| Quereux et al. (3; ref. 31) | Quereux et al. (57) | France, 2009 | Cohort | Self-completion of questionnaire and examination of patients at high risk | Consecutive patients > 18 visiting their doctor | 1,039 (43%)e | Efficiency 11.54 (P = 0.0016)f | |||
aOf 1,751 who agreed to take part, 1,024 (58%) were subsequently excluded as they were not eligible.
bBased on both development and test populations.
cThe initial response rate to the questionnaire was 66%. Of those who responded, 388 (26%) attended for a skin examination.
dOf 1,500 patients agreeing to take part, 42 (2.8%) were excluded as they were not eligible and 100 (6.7%) for incomplete data.
eA total of 7,953 completed the questionnaire while visiting their GPs; 2,404 were high risk and 1,039 (43%) of those consulted a dermatologist. Of those 95 had a biopsy and a melanoma was found in 10.
fThe interpretation of this is that to detect a new case of melanoma it is necessary to screen 11.54 times fewer patients than with nontargeted screening.
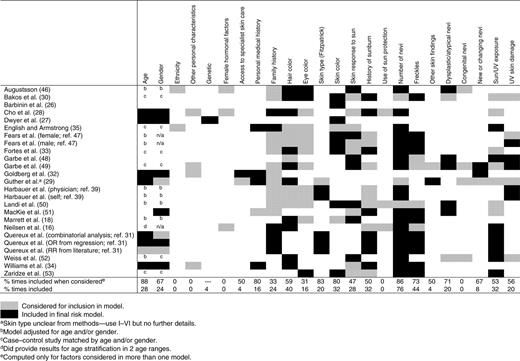
Between them, the 25 risk prediction models considered 144 different possible risk factors (Supplementary Table S3). These included 18 different measures of number of nevi, 26 of sun/UV exposure, and 14 of history of sunburn. There were also multiple definitions of dysplastic nevi with each research group using a different definition. Categorizing the different risk factors, as shown in Supplementary Table S3, allowed comparison of those considered and included in each of the risk models (Table 3). This shows that the risk factors most frequently included in the models are (in order of frequency) number of nevi, freckles, hair color, skin color, history of sunburn, and sun/UV exposure. The risk factors most likely to remain in the final model after consideration are age, number of nevi, skin type, skin color, personal history of skin cancer, and freckles. Ethnicity, other personal characteristics, such as sociodemographic measures, female hormonal factors, use of sun protection, and congenital nevi were not included in any of the final models, and a family history of skin cancer and eye color were included in the final model in less than 1 in 5 times they were considered.
Discussion
Principal findings
This is the first systematic review of risk prediction models for melanoma. It shows that multiple risk models exist and that they have the potential to identify individuals at higher risk of melanoma. Comparisons between the different models are difficult due to the lack of validation studies and heterogeneity in choice and definition of variables. Despite this, however, we show that most include well-established risk factors and the AUROC of a summary ROC curve is comparable with those for other cancers, such as breast cancer (0.716–0.762; ref. 36) and colon cancer (0.61–0.74; ref. 37). There was also little difference in model performance between those scores suitable for self-assessment and those requiring a health care professional, suggesting potential for use at a population level to identify people at higher risk of melanoma.
Strengths and limitations
The main strengths of this review are the use of broad inclusion criteria and the systematic search of multiple databases not limited by language. This approach enabled us to identify published risk models even when developing the risk model had not been the primary aim of the study and in doing so reduces the risk of selection bias. While we cannot exclude publication bias, we also expect this to be minimal because of the exploratory nature of many of the studies and the absence of performance data.
As with most systematic reviews, the main limitation is the quality of the published data. Notably, in this review, it was difficult to perform direct comparisons of the risk models due to the lack of validation studies for most of the risk models. The majority of studies also gave no indication of how the authors decided which risk factors to consider for inclusion in the model and 144 different risk factors were considered with varying definitions. In addition, many of the risk factors are subjective in nature and subject to recall bias, which is likely to overestimate the performance of those models developed from case control studies, and only 4 included blinding of the investigator to melanoma status. By presenting all the risk models together for the first time, however, we are able to demonstrate this heterogeneity while making comparisons where possible.
Evaluation of the risk models
The 25 risk models differ in the risk factors included, the method of administration, and their performance. Most contain established risk factors for melanoma, however, there was considerable variation among the definitions and measures used. In some cases, notably history of sunburn and sun/UV exposure, this likely reflects the difficulty measuring exposure to the risk factor, both due to its subjective nature and the need to recall events in the past. This is in contrast to more objective and consistent measures, such as eye color, skin type, or hair color for which many fewer variations were seen. In other cases, particularly number of nevi and atypical and dysplastic nevi, the range of definitions probably reflects ongoing uncertainty within the literature and the controversy around a nonhistologic diagnosis of an atypical or dysplastic nevus (38). In all cases, however, it demonstrates the large number of variables in use within the field. While it is unlikely that a single measure of each risk factor will be appropriate for all situations, increased consistency would allow more meaningful comparisons in future research.
With such a large number of risk factors considered, it is perhaps not surprising that the models differ widely in the risk factors included. Most include a measure of number of nevi and skin type or color and either include or adjust for age and gender but beyond that it is difficult to make generalizations.
Performance measures were only available for 16 models in the development population and 2 in external populations. Despite the variations already described, however, the accuracy, measured by the sensitivity and specificity, is consistent across them. By virtue of the cutoff values set by the authors, some have higher specificity and lower sensitivity (18, 34, 35, 39) while others have higher sensitivity and lower specificity (26, 31, 33). The summary ROC curve, however, shows that, despite including a range of different variables, there is very little heterogeneity in the predictive ability of the models with the variations in sensitivity and specificity reflecting different cutoff values. One reason for this may be that there is a group of core risk factors responsible for most of the increased risk. Because of the range of factors included in the different models, however, it is not possible to identify those from the available studies.
The discrimination of the models, as measured by the AUROC, compares favorably with risk models used for other cancers, including breast cancer with AUROCs of 0.716 to 0.762 (36) and colon cancer with AUROCs of 0.61 to 0.74 (37). Care must be taken when making such comparisons, however, as many of these have been developed and validated in large cohort studies, while the majority of melanoma risk models have been developed from case–control studies with up to 60% prevalence of melanoma which will inflate their performance through spectrum bias.
Evaluation of individual risk factors
While evaluation of individual melanoma risk factors was not the primary aim of this study, by including only studies that used a statistical method to develop a risk model and extracting the number of times a risk factor was included in the final model when it was considered, the results of our analysis confirm the importance of several established risk factors for melanoma (23–25). These include age, number of nevi, skin type and color, personal history of melanoma or nonmelanocytic skin cancer, freckles, dysplastic nevi, and hair color. Sun exposure, history of sunburn, and skin response to the sun were also included in many of the final models but only half the times they were considered (53%, 50%, and 47% respectively), perhaps reflecting their subjective nature and risk of recall bias. Eye color was also only included in 4 of the 13 models in which it was considered and this is likely to be due to known correlation between hair color, eye color, freckles, and skin color (23).
An unexpected finding was the absence of family history in many of the models. It was considered in 18 of the models but only remained in the final score in 6. This differs from earlier studies in which approximately 10% of cases of melanoma have reported heredity (23, 40). It may be that other phenotypic markers which remain in the risk model are strongly correlated with family history or may simply reflect the very low incidence of true familial melanoma in the melanoma population. Some other risk factors, including, for example, genetic factors, ethnicity, and female hormone factors, were also not considered by very many of the models and so their potential importance may be underestimated.
Implications for clinicians and policy makers
This review shows that multiple risk models for prediction of the development of melanoma exist and that they have the potential to identify individuals at higher risk of melanoma. Clinicians will be interested to see the range and relative performance of different risk models. However, all the risk scores were developed to predict risk of future disease rather than undiagnosed prevalent disease. Consequently, the results of this review will be of particular relevance to policy makers interested in the potential for using risk scores among asymptomatic people to identify a subset of the population for whom targeted screening, surveillance, or educational programs could be offered to reduce the morbidity and mortality from melanoma.
As English and Armstrong (35) point out, if a screening program is to be directed towards a high risk group and is to have an impact on the disease as a whole, 3 criteria must be satisfied in addition to those for all screening programs (41): people at high risk of the disease must be readily identifiable; those identified as being at high risk must form a large proportion of all patients who develop the disease; and this proportion must be substantially larger than the proportion of the whole population that constitutes the group at high risk. When assessed against these 3 criteria, this review confirms that risk models exist which could be used to identify a group at higher risk of melanoma. First, a number of risk models exist for which patient self-assessment is feasible and so they could be undertaken in clinical waiting rooms or via online platforms (16, 18, 28, 30–32, 34, 35, 39). Second, those models that provide values for sensitivity and specificity suggest that screening could identify a high risk group containing between 25% and 89% of people expected to develop melanoma and, third, that this high-risk group would comprise between 10% and 55% of the population. These ranges are wide due to the variation in cutoff values selected in each study and reflect the trade-off between sensitivity and specificity. For example, from the summary ROC, choosing a risk score with a specificity of 50% when 50% of the population would be classified as higher risk, sensitivity is around 80%, so 80% of melanomas would be detected in that higher risk group. Choosing instead a score with a specificity of 80% when 20% would identified as high risk, the sensitivity decreases to around 50%, so only 50% of cases would be detected.
Some, including Fortes (33), believe that as melanoma can be a fatal disease but referral to a dermatologist and excision or biopsy is relatively benign, it is better to give priority to sensitivity over specificity as the inclusion of false-positive cases may be less detrimental than false-negatives. However, the use of a risk score that identifies 50% of the population as higher risk is limited, and any screening of asymptomatic people has considerable implications in terms of health care costs and both physical and psychological consequences. Several previous studies have estimated the cost-effectiveness of various melanoma screening strategies. One-off screening of a white population of all ages at average risk by a dermatologist has been shown to cost $172,276 per year-of-life-saved (YLS; ref. 42) but this cost decreases dramatically when screening is targeted to higher risk populations, defined variously by age, family history, or phenotypic characteristics (10, 11, 43). While a full economic analysis is beyond the scope of this review, the risk scores described are able to identify higher risk groups with greater discriminatory ability and accuracy than age, family history, or phenotypic characteristics alone and so any screening program based on one of the risk models is likely to be even more cost-effective.
Implications for future research
The finding that many of the models have similar performance characteristics despite the wide range of different variables included suggests that developing further models based on current known risk factors is unlikely to benefit the field. As advances are made into genes that play a role in the susceptibility of melanoma (44, 45), development of new risk models incorporating genetic information may improve the discriminatory ability. Until then, further research should focus on validating existing models in different populations and assessing the costs, feasibility, acceptability, and adverse consequences of applying these models.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Acknowledgments
The authors thank Isla Kuhn, Reader Services Librarian, University of Cambridge Medical Library, for her help developing the search strategy and Professor Simon Griffin and the MelaTools program's expert panel (Drs. Katharine Acland, Nigel Burrows, Pippa Corrie, and Mr. Per Hall) for their helpful comments on the manuscript.
Grant Support
This report is independent research arising from a Clinician Scientist award supported by the National Institute for Health Research (RG 68235). J.A. Usher-Smith is funded by a National Institute for Health Research Clinical Lectureship.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.